Neuromyelitis Optics Therapy Market Size and Forecast – 2026 – 2033
The Global Neuromyelitis Optics Therapy Market size is estimated to be valued at USD 1.45 billion in 2026 and is expected to reach USD 3.12 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 11.8% from 2026 to 2033.
Global Neuromyelitis Optics Therapy Market Overview
Neuromyelitis optics therapy products include pharmaceutical treatments used to manage autoimmune inflammation of the optic nerves and spinal cord. These therapies focus on reducing immune-mediated damage, preventing disease relapses, and preserving neurological function. Products include immunosuppressive agents, monoclonal antibodies, and infusion-based treatments. They are prescribed and administered under specialist supervision due to the complexity of the condition.
Key Takeaways
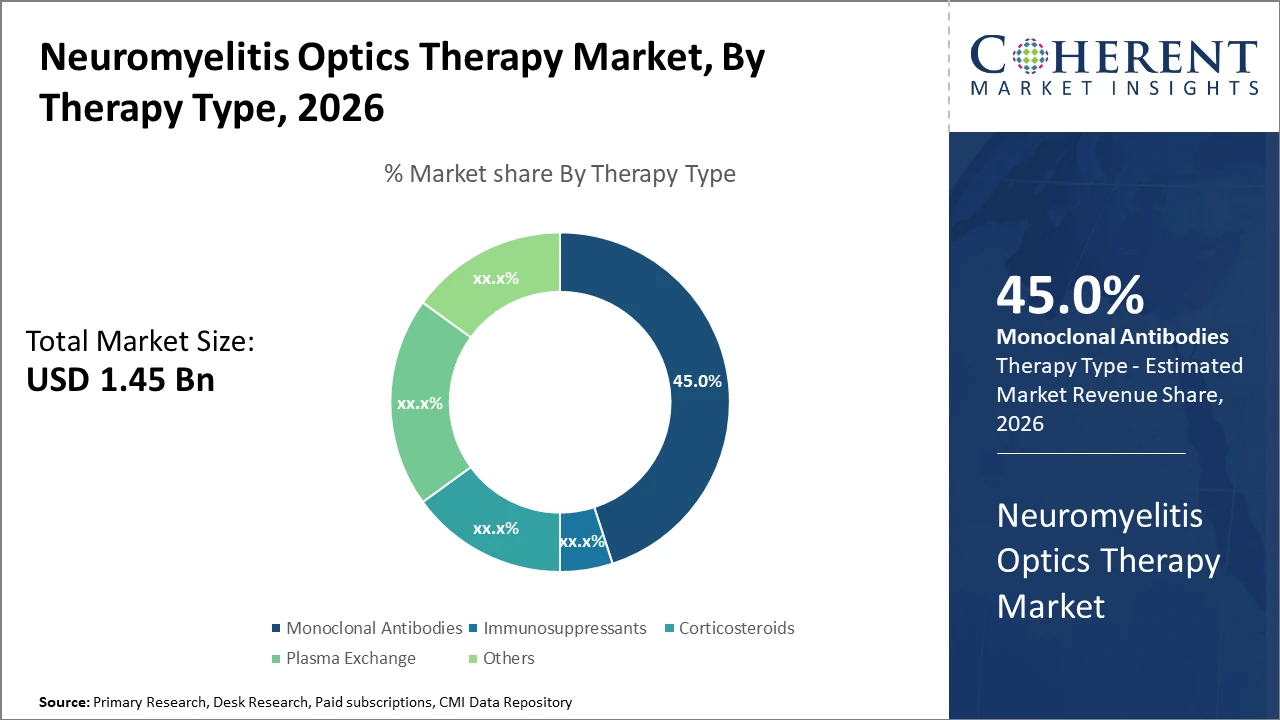
The monoclonal antibodies segment dominates the therapy type category with a 45% market share, driven by robust clinical efficacy and expanded regulatory endorsements in recent years.
Relapse prevention applications lead the market, accounting for 52% of total revenue, reflecting higher adoption rates fueled by clinical trials demonstrating superior outcomes.
Hospitals and specialty treatment centers continue to be the principal end-users, benefiting from growing infrastructure investments and enhanced treatment protocols.
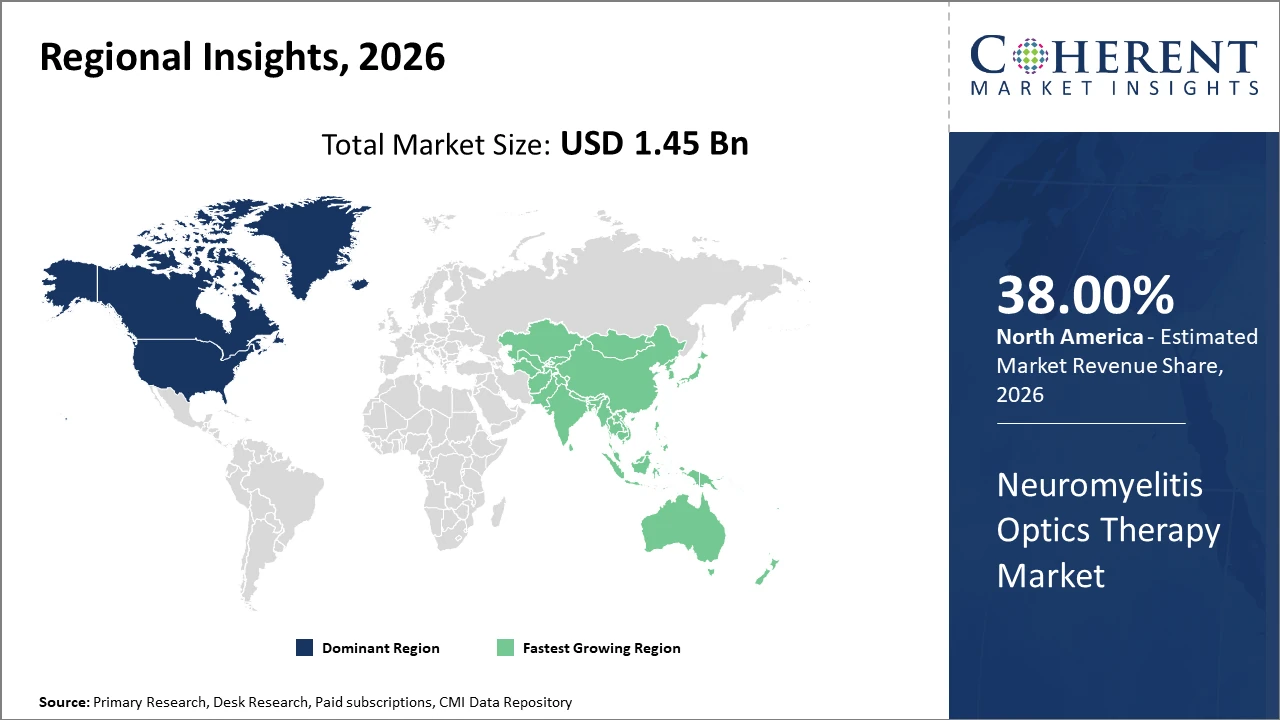
Regionally, North America sustains dominance with over 38% market share backed by a well-established healthcare ecosystem and significant R&D expenditures.
Asia Pacific is the fastest-growing region, witnessing a CAGR exceeding 14%, propelled by expanding patient populations and favorable government policies promoting access to innovative therapies.
Neuromyelitis Optics Therapy Market Segmentation Analysis
To learn more about this report, Download Free Sample
Neuromyelitis Optics Therapy Market Insights, By Therapy Type
Monoclonal Antibodies dominate the market share. This segment benefits from strong clinical efficacy in relapse prevention, supported by accelerated approvals and adoption in both developed and emerging markets. The subsegment’s dominance stems from therapies that specifically target pathogenic antibodies, thereby significantly reducing disease progression. Immunosuppressants, while still significant, are experiencing slower growth due to side effect profiles and emerging safer alternatives. Corticosteroids mainly play a role in acute relapse management but face limitations for long-term use. Plasma exchange retains niche relevance in severe cases.
Neuromyelitis Optics Therapy Market Insights, By Application
Relapse Prevention dominates market share. The focus on relapses arises from their critical impact on patient quality of life, healthcare costs, and disability progression. The emergence of highly effective biologics has transformed relapse prevention strategies, causing significant shifts in treatment guidelines since 2023. Acute Relapse Treatment remains vital, especially in hospital settings, but its growth is limited compared to preventive interventions. Maintenance Therapy is increasingly gaining traction as long-term disease control becomes a focal point for clinicians.
Neuromyelitis Optics Therapy Market Insights, By End-User
Hospitals and Clinics constitute the largest segment due to well-established neurology departments equipped for advanced diagnostics and acute care management. Specialty Treatment Centers represent the fastest-growing subsegment, propelled by increased specialization and patient-centric care models offering personalized therapy regimens. Homecare Settings are emerging as convenient alternatives, supported by telemedicine and remote monitoring technologies, enhancing patient adherence and reducing clinical visits.
Neuromyelitis Optics Therapy Market Trends
The Neuromyelitis Optics Therapy market continues to evolve with precision medicine taking center stage, as evidenced by the introduction of biomarker-specific treatments in 2025, improving therapeutic outcomes.
Combination therapies have shifted treatment paradigms, with real-world evidence from neurology centers in Europe showing enhanced relapse control.
Digital health platforms have emerged as transformative tools facilitating improved patient monitoring, promoting adherence, and reducing relapse rates.
For instance, in late 2025, a notable pharma-tech collaboration launched a mobile app to support remote patient management, underlining tech’s critical role in advancing therapy effectiveness.
Neuromyelitis Optics Therapy Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Neuromyelitis Optics Therapy Market Analysis and Trends
In North America, the dominance in the Neuromyelitis Optics Therapy market is underscored by a well-established healthcare infrastructure, extensive patient awareness programs, and significant investments in R&D. Approximately 38% of the market share originates here, propelled by a robust pipeline of innovative biologics and favorable reimbursement frameworks. Key players in the region are continuously expanding product portfolios and optimizing clinical trial networks, ensuring sustained leadership in market share and revenue generation.
Asia Pacific Neuromyelitis Optics Therapy Market Analysis and Trends
Meanwhile, the Asia Pacific region exhibits the fastest growth, with a CAGR surpassing 14%. This surge is driven by increasing NMOSD prevalence, expanded healthcare access, and proactive government initiatives supporting biologic therapies' approval and reimbursement. China and India notably spearhead growth due to rising patient awareness and healthcare expenditure increases. Regional companies are also enhancing manufacturing capacities and export activities, further accelerating market penetration.
Neuromyelitis Optics Therapy Market Outlook for Key Countries
USA Neuromyelitis Optics Therapy Market Analysis and Trends
The USA's Neuromyelitis Optics Therapy market benefits from advanced healthcare infrastructure and a strong presence of leading pharmaceutical companies such as Alexion Pharmaceuticals and Regeneron. In 2025, the country witnessed a 15% rise in newly diagnosed NMOSD cases, further stimulating demand for innovative therapies. The U.S. regulatory environment expedited approval processes for next-generation monoclonal antibodies, enhancing market revenue streams. Additionally, substantial government and private-sector funding have increased clinical research activities, facilitating early adoption of novel therapies and solidifying the USA’s pivotal role in market dynamics.
Japan Neuromyelitis Optics Therapy Market Analysis and Trends
Japan's Neuromyelitis Optics Therapy market exhibits high potential driven by demographic trends such as an aging population and increasing autoimmune disease awareness. Recent policy reforms in 2025 improved insurance coverage for monoclonal antibody treatments, boosting therapy accessibility. Leading companies, including Mitsubishi Tanabe Pharma and Eisai, have intensified market penetration through local partnerships and tailored therapies suited for the patient population. Japan’s proactive healthcare ecosystem and focus on innovation represent critical factors fueling market growth and competitive positioning.
Analyst Opinion
The growing incidence of NMOSD, particularly in Asia Pacific and North America, drives an increased demand for advanced therapies. Recent clinical registries from 2025 indicate a global incidence rate nearing 1.5 per 100,000 individuals, with diagnostic improvements fueling early treatment uptake. For example, the U.S. recorded a 12% year-on-year increase in patients initiating biologic treatment in 2025.
Pricing and reimbursement dynamics substantially influence market revenue. In 2025, government-backed insurance programs in Europe expanded coverage for NMOSD therapies, contributing to a 20% increase in therapy adoption while maintaining stable average treatment costs. This underscores the role of healthcare policies in scaling market revenue despite high therapy prices.
Demand-side factors highlight off-label use expansion and combination therapies. Usage of complement inhibitors beyond primary NMOSD indications rose by 8.3% in 2023 within leading neurology centers, reflecting diversified clinical applications that bolster market scope and business growth. This multipronged approach underpins a broader range of use cases bolstering market share.
Supply chains have adapted swiftly, with manufacturers increasing production capacity by more than 15% in 2025 to meet global demand surges. Notable exports from Asia Pacific to Latin America have streamlined access, achieving a 25% rise in international shipments of monoclonal antibodies used in NMOSD therapy. This capacity enhancement aids in mitigating market challenges such as regional product shortages.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 1.45 billion |
| Historical Data for: | 2021 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 11.8% | 2033 Value Projection: | USD 3.12 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Viela Bio, Inc., UCB S.A., CSL Limited, BioMarin Pharmaceutical Inc., Regeneron Pharmaceuticals, Inc., Eisai Co., Ltd., Sanofi S.A., Biogen Inc., Genentech, Inc., Amgen Inc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Neuromyelitis Optics Therapy Market Growth Factors
The expanding prevalence of NMOSD, particularly in Asia Pacific and North America, remains a pivotal driver, with patient awareness campaigns increasing early diagnosis rates by up to 15% in 2025. Novel biologics and therapeutic combinations are revolutionizing treatment paradigms, as evidenced by trial data from 2023 demonstrating a 40% reduction in relapse rates with next-gen monoclonal antibodies. Increasing healthcare infrastructure investments in developing nations are broadening therapy accessibility, thereby uplifting market revenue significantly. Additionally, favorable regulatory approvals expedited by breakthrough therapy designations in 2025 contributed to faster market entry for innovative therapies, directly impacting market growth positively.
Neuromyelitis Optics Therapy Market Development
In August 2020, Genentech’s Enspryng® (satralizumab) received U.S. FDA approval as the first subcutaneous treatment for adults with aquaporin-4 (AQP4) antibody-positive neuromyelitis optica spectrum disorder (NMOSD). The approval marked a major advancement in NMOSD management by enabling patients to self-administer therapy at home, reducing reliance on infusion centers while demonstrating significant relapse-reduction efficacy in pivotal clinical trials.
In March 2024, AstraZeneca’s Ultomiris® (ravulizumab-cwvz) was approved by the U.S. FDA for the treatment of AQP4 antibody-positive NMOSD, expanding therapeutic options in this rare autoimmune disease. As a long-acting C5 complement inhibitor, Ultomiris offers extended dosing intervals compared with earlier complement therapies, helping to lower treatment burden while maintaining strong protection against disease relapses.
Key Players
Leading Companies of the Market
Viela Bio, Inc.
UCB S.A.
CSL Limited
BioMarin Pharmaceutical Inc.
Regeneron Pharmaceuticals, Inc.
Eisai Co., Ltd.
Sanofi S.A.
Biogen Inc.
Genentech, Inc.
Amgen Inc.
Several leading companies are intensifying R&D investments to develop next-generation monoclonal antibodies, with Horizon Therapeutics notably achieving a 17% revenue increase in 2025 linked to new drug approvals for NMOSD. Alexion Pharmaceuticals leveraged strategic alliances to enhance global distribution, resulting in a 22% uplift in market penetration in emerging regions. Furthermore, Regeneron’s focus on biosimilar development yielded competitive pricing strategies that have begun disrupting established market dynamics, enhancing the overall market growth strategies efficacy.
Neuromyelitis Optics Therapy Market Future Outlook
The future of NMOSD therapy is characterized by expanding biologic innovation, improved diagnostic precision, and more personalized treatment regimens. Emerging therapies will focus on more selective immune modulation with fewer adverse effects, potentially including small-molecule agents and combination approaches. Advances in biomarkers and genetic profiling will enable better patient stratification and earlier intervention. Increasing awareness among clinicians and improved access to specialty neurologic care will further support treatment uptake. Global clinical trials and real-world evidence will continue to inform best practices, making NMOSD therapy a model for targeted autoimmune disorder management.
Neuromyelitis Optics Therapy Market Historical Analysis
Neuromyelitis optic therapy has historically faced challenges due to the rarity and complexity of the disease, which affects the optic nerves and spinal cord. Early treatment strategies focused on non-specific immunosuppression with corticosteroids and plasmapheresis, often with variable outcomes. The identification of aquaporin-4 autoantibodies in the early 2000s and subsequent recognition of NMOSD as a distinct clinical entity fundamentally changed therapeutic approaches. Targeted biologic therapies emerged in the late 2010s, offering significant improvements in relapse prevention and disease management. Regulatory approvals for monoclonal antibodies specifically designed to modulate immune activity reinforced the legitimacy of precision therapy for NMOSD and stimulated research investment.
Sources
Primary Research Interviews:
Neurologists
Immunologists
Clinical Researchers
Specialty Pharmacists
Healthcare Policy Experts
Databases:
NIH Neurological Reports
WHO Autoimmune Data
OECD Health Statistics
Magazines:
Neurology Today
Rare Disease Advisor
BioPharma Insight
Clinical Neurology News
MedPage Today
Journals:
Neurology Journal
Multiple Sclerosis Journal
Journal of Neuroimmunology
CNS Drugs
Autoimmune Disease Journal
Newspapers:
Financial Times (Health)
Reuters Healthcare
The Guardian (Science)
New York Times (Health)
Bloomberg
Associations:
American Academy of Neurology
National Organization for Rare Disorders
European Academy of Neurology
Autoimmune Association
World Health Organization
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients