There are three types of Neurofibromatosis; Neurofibromatosis type 1, Neurofibromatosis type 2, and Schwannomatosis. Neurofibromatosis type (NF) 1 is a common monogenic disorder of neurocutaneous tissue growth that arises secondary to mutations in the tumor suppressor gene NF1. It features an autosomal dominant pattern of inheritance. Individuals with NF1 typically have an increased predisposition to a variety of benign and malignant tumors and develop neurofibromas, and axillary and inguinal freckling. Neurofibromatosis 2 (NF2) is less common than NF1. Common signs and symptoms of NF 2 include benign, slow-growing tumors affecting the cranial, spinal, and peripheral nerves, as well as the meninges. KOSELUGO- selumetinib is the only drug available for the treatment of Neurofibromatosis Type-1. Moreover, some drugs such as bevacizumab are also used as off-label for the treatment of Neurofibromatosis
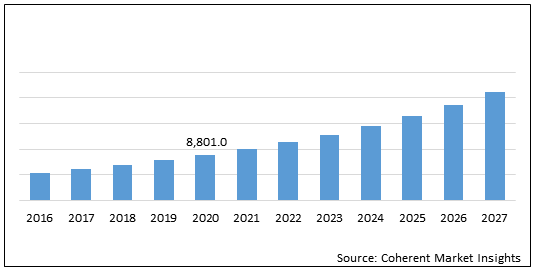
The global neurofibromatosis treatment drugs market is estimated to be valued at US$ 8,801.0 million in 2020 and is expected to exhibit a CAGR of 13.40% during the forecast period (2020-2027).
Figure 1. Global Neurofibromatosis Treatment Drugs Market Value (US$ Mn)
To learn more about this report, Download Free Sample
Global Neurofibromatosis Treatment Drugs Market: Drivers
Increasing focus of major players on research and development of novel therapies for treating neurofibromatosis. Moreover, clinical studies are well supported by regulatory authorities which is expected to drive the growth of the global neurofibromatosis treatment drugs market. For instance, in October 2019, SpringWorks Therapeutics, Inc., a clinical-stage biopharmaceutical company focused on developing life-changing medicines for patients with severe rare diseases and cancer, initiated the Phase 2b ReNeu clinical trial to evaluate the mirdametinib (formerly PD-0325901), an oral, small molecule designed to inhibit MEK1 and MEK2, in children and adult patients with neurofibromatosis type 1 (NF1)-associated plexiform neurofibromas (NF1-PN). In July 2019, the European Commission granted Orphan Drug Designation for SpringWorks Therapeutics’ mirdametinib (formerly PD-0325901), an oral, small molecule inhibitor of MEK1 and MEK2, for the treatment of neurofibromatosis type 1 (NF1).
Neurofibromatosis Treatment Drugs Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2019 | Market Size in 2019: | US$ 8,801.0 Mn |
| Historical Data for: | 2016 to 2019 | Forecast Period: | 2020 to 2027 |
| Forecast Period 2020 to 2027 CAGR: | 13.4% | 2027 Value Projection: | US$ 21,223.7 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
AstraZeneca Plc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Neurofibromatosis Treatment Drugs Market – Impact of Coronavirus (COVID-19) Pandemic
The coronavirus (COVID 19) pandemic and lockdown in various countries across the globe have impacted the financial status of businesses in all sectors. Private healthcare is one such sector, which has been majorly impacted by the COVID-19 pandemic.
The lockdown in various countries due to the pandemic has placed an economic burden on the private healthcare sector.
Moreover, the coronavirus pandemic has hampered the development, production, and supply of healthcare products (medical devices and medicinal products) and affected growth of healthcare businesses of various companies across the globe.
The lockdowns have led to closure of industrial establishments, except manufacturing of essential commodities, and disrupted supply chains.
The COVID-19 pandemic has affected the economy in three main ways; 1) by directly affecting the production and demand; 2) by creating disruptions in distribution channels; and 3) by its financial impact on firms and financial markets
As a result, the impact of coronavirus (COVID-19) pandemic is also expected to limit growth of the global neurofibromatosis treatment drugs market during the forecast period.
Figure 2. Global Neurofibromatosis Treatment Drugs Market Value (US$ Mn), by Region, 2020
To learn more about this report, Download Free Sample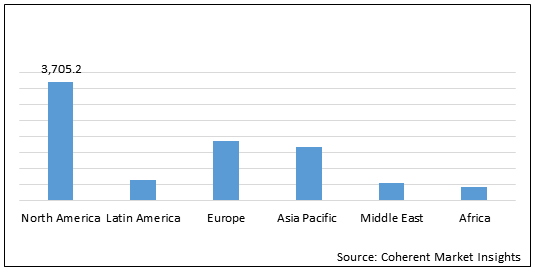
North America neurofibromatosis treatment drugs market is expected to show significant growth during the forecast period owing to presence of key players and research institutes who are involved in clinical trial programmes. For instance, on November 28, 2017, the University of Alabama at Birmingham initiated phase 2 clinical study on Binimetinib in children and adults with nf1 plexiform neurofibromas. On February 18, 2020, the University of Alabama at Birmingham initiated phase 2 clinical trial of crizotinib for children and adults with neurofibromatosis type 2 and progressive vestibular.
Key Players
Major Player operating in the global neurofibromatosis treatment drugs market is AstraZeneca Plc.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients