Neuroendocrine Tumor Treatment Market is estimated to be valued at USD 3.65 Bn in 2025 and is expected to reach USD 5.56 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 6.2% from 2025 to 2032.
Analysts’ Views on the Global Neuroendocrine Tumor Treatment Market:
Increasing grants for research and development activities for neuroendocrine tumor at research institutes is expected to drive the growth of the global neuroendocrine tumor treatment market over the forecast period. For instance, in November 2022, a five-year of US$ 3.3 million grant from the National Institutes of Health (NIH) was given to the Institute of Molecular Medicine at McGovern Medical School to research receptor-targeted fluorescence-guided surgery for pancreatic neuroendocrine tumors. Researchers will be able to undertake a Phase 1 clinical study with a fluorescent contrast agent that can detect neuroendocrine tumors in real-time during surgery due to funding for the project, "Receptor-targeted Fluorescence-Guided Surgery in Pancreatic Neuroendocrine Tumors." The Azhdarinia lab focuses on the creation of compounds that can be employed as contrast agents in nuclear imaging and fluorescence-guided surgery. Lab researchers also specialize in targeted medicine delivery straight to tumors.
Figure 1. Global Neuroendocrine Tumor Treatment Market Share (%), By Drug Class, 2025
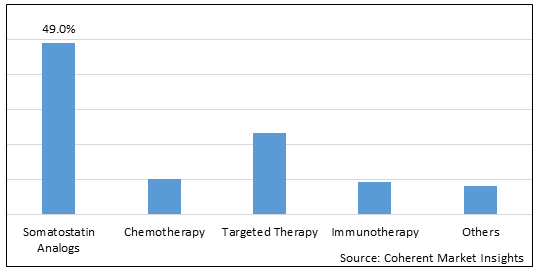
To learn more about this report, Download Free Sample
Global Neuroendocrine Tumor Treatment Market - Drivers
Figure 2. Global Neuroendocrine Tumor Treatment Market Share (%), By Region, 2023
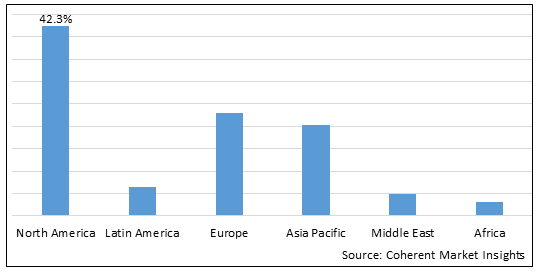
To learn more about this report, Download Free Sample
Global Neuroendocrine Tumor Treatment Market - Regional Analysis
Among regions, North America is estimated to hold a dominant position in the global neuroendocrine tumor treatment market over the forecast period due to key market players being focused on adopting strategies such as acquisition and merger to enhance their market share. For instance, on February 6, 2025, Isoray, Inc., a medical technology company pioneering in advanced treatment applications and devices to deliver targeted internal radiation treatments for cancers, announced the completion of the merger with Viewpoint Molecular Targeting, Inc., a radiopharmaceutical company that is developing precision oncology therapeutics and complementary diagnostic imaging agents. Both companies will focus on the advancement of cancer treatments by using radiation, radiopharmaceuticals, and imaging technologies so that precision, targeted medical doses are delivered directly to cancer patient tumor sites. This merger will further help the company expand its product portfolio.
Neuroendocrine Tumor Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 3.65 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.2% | 2032 Value Projection: | USD 5.56 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Pfizer Inc, Novartis AG, Ispen, Advanced Accelerator Applications, Tarveda Therapeutics, Progenics Pharmaceuticals, Inc., Hutchison Medipharma Limited, Dauntless Pharmaceuticals Inc., and Exelixis, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Neuroendocrine Tumor Treatment Market - Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization (WHO) declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, the U.A.E., Egypt, and others, faced problems regarding transportation of products from one place to another.
The COVID-19 pandemic also had a negative impact on the global neuroendocrine tumor treatment market. For instance, according to a article published in January 2021, titled "Impact of the COVID-19 pandemic on neuroendocrine tumor services in England" in the journal Endocrine, there were particular worries about the specialized tumor treatment services during the COVID-19 pandemic. According to the same article, the major interruption in specialist neuroendocrine treatment services included noticeably longer wait times for both new and follow-up consultations, with follow-up appointments being delayed the most.
Global Neuroendocrine Tumor Treatment Market Segmentation:
The global neuroendocrine tumor treatment market is segmented into drug class, indication, and distribution channel.
Among all the segmentation, the drug class segment is expected to have high potential in the global neuroendocrine tumor treatment market over the forecast period, and this is attributed to the increasing product approvals from the regulatory authorities. For instance, in December 2021, Cipla Limited, an India-based multinational pharmaceutical company, and its subsidiary Cipla USA, Inc. announced that they had received final approval for their lanreotide injection from the U.S. Food and Drug Administration (FDA). Lanreotide is a somatostatin analogue. It can be used for the treatment of patients with acromegaly and gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
Global Neuroendocrine Tumor Treatment Market - Cross Sectional Analysis:
Among the indication segment, gastrointestinal NET segment is expected to dominate the market in the Europe region, owing to the increasing research and development activities. For instance, the Journal of Cancer Research and Clinical Oncology 2022 published a retrospective cross-sectional study to identify gastrointestinal (non-pancreatic) neuroendocrine tumors. The results indicated that the majority of patients had tumors that originated in the small intestine; however, patients from Germany had a little overrepresented NET of the large intestine and a significantly underrepresented NET of the stomach. More than 80% of patients had stage IV disease at the time of diagnosis. Regarding tumor histology, most tumors showed a G2 (Grade 2) tumor. G3 grading was found in 40.9% of patients in Germany. Thus, Germany-based people are more susceptible to gastrointestinal neuroendocrine tumors than the rest of the European countries.
Global Neuroendocrine Tumor Treatment Market: Key Developments
Global Neuroendocrine Tumor Treatment Market: Key Trends
Adoption of growth strategies such as product approval, launch, etc.
Key market players are focused on adopting various growth strategies, such as product approval, launch and others, which in turn is expected to boost market growth over the forecast period. For instance, in October 2022, ITM Isotype Technologies Munich SE, a pharmaceutical company, announced that the U.S. Food and Drug Administration (FDA) had granted a fast track designation to Lu-edotreotide (ITM-11) for use as a potential therapeutic option in patients with gastroenteropancreatic neuroendocrine tumors (GEP-NETs). ITM-11 is comprised of the octreotide-derived somatostatin analog edreotide (Dotatoc) and no-carrier lutetium-177 chloride, which is a beta-emitting therapeutic radioisotope.
Global Neuroendocrine Tumor Treatment Market: Restraint
Global Neuroendocrine Tumor Treatment Market - Key Players
The major players operating in the global neuroendocrine tumor treatment market include Pfizer Inc, Novartis AG, Ispen, Advanced Accelerator Applications, Tarveda Therapeutics, Progenics Pharmaceuticals, Inc., Hutchison Medipharma Limited, Dauntless Pharmaceuticals Inc., and Exelixis, Inc.
Definition: A neuroendocrine tumor treatment refers to the various methods used to manage and treat neuroendocrine tumors, which are rare, slow-growing tumors that originate in the body's neuroendocrine cells. These cells are responsible for producing hormones, so these tumors can sometimes lead to an overproduction of hormones in the body.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients