Nephrology and urology devices are used for treatment of kidney and urinary system related problems such as chronic kidney disease, kidney stone, benign prostatic hyperplasia, urinary incontinence and pelvic organs prolapse. There are multiple options available for treatment of urology diseases and physicians suggest suitable options depending upon health conditions of patients and stage of disease.
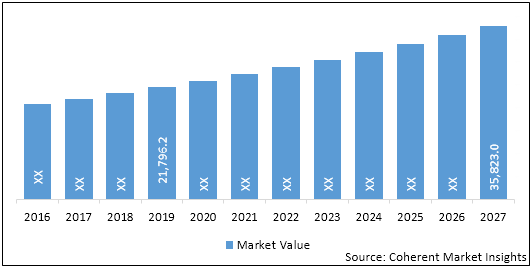
The global nephrology and urology devices market is estimated to account for US$ 23,192.8 Mn in terms of value in 2020 and is expected to reach US$ 35,823.0 Mn by the end of 2027.
Global Nephrology and Urology Devices Market: Drivers
Increasing prevalence of chronic kidney diseases is expected to propel growth of the global nephrology and urology devices market over the forecast period. For instance, according to the study, ‘Risk Factors and Rate of Progression of CKD in Children’, published in June 2019 in Kidney International Reports, 38% children experienced progression of chronic kidney disease within 2 years of follow-up.
Moreover, increasing preference for minimally invasive and non-invasive treatment procedures is also expected to boost demand for nephrology and urology devices. Minimally invasive and non-invasive surgical treatments for urology diseases such as incontinence, urinary stone, and benign prostatic hyperplasia (BPH) as it considerably reduces the treatment cost and time over conventional surgical methods. Advanced treatment methods used for urinary stone treatments such as extracorporeal shock wave lithotripsy (ESWL) and laser stone surgery increases treatment efficacy and speed and minimizes hospitalization days, which reduce the financial burden from the patient.
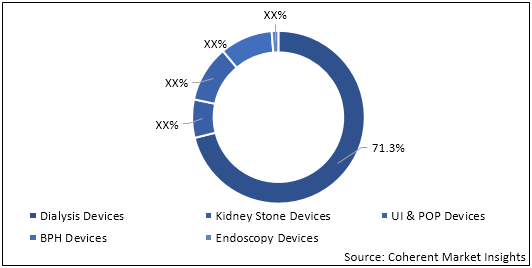
Dialysis Devices held dominant position in the global nephrology and urology devices market in 2019, accounting for 71.3% share in terms of value.
Figure 1. Global Nephrology and Urology Devices Market Share (%), by Value, by Device Type, 2019
To learn more about this report, Download Free Sample
Global Nephrology and Urology Devices Market: Restraints
High installation prices of device for lithotripters and advanced machines is expected to hinder growth of the global nephrology and urology devices market. Advanced technology devices such as ESWL, dialysis machines, and endoscopy devices are not affordable for small and many midsized healthcare providers. Lithotripter machines integrated with localization systems and lithotripsy table cost US$ 380,000 to US$ 450,000 in Europe. Installation of low budget lithotripsy machine costs at an average US$ 250,000, whereas in India, installation of lithotripters machines cost at an average US$ 350,000.
Moreover, product recalls are also expected to hinder growth of the global nephrology and urology devices market. For instance, in May 2020, The U.S. Food and Drug Administration designated Applied Medical’s recall of three latex balloon catheters as a class 1 recall, the most serious kind, which will affect 19,400 catheters (Python embolectomy catheters, Bard embolectomy catheters, OTW Latis cleaning catheters).
Nephrology and Urology Devices Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2019 | Market Size in 2019: | US$ 21,796.2 Mn |
| Historical Data for: | 2016 to 2019 | Forecast Period: | 2020 to 2027 |
| Forecast Period 2020 to 2027 CAGR: | 6.4% | 2027 Value Projection: | US$ 35,823.0 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Fresenius Medical Care, Baxter International Inc., Asahi Kasei Corp., BD, Cook Group Incorporated, Dornier MedTech, Nikkiso Co. Ltd., NxStage Medical Inc., Nipro Corporation, Terumo Corporation, B Braun Group, Medtronic Plc, Awak Technologies, and Rockwell Medical Technologies, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Nephrology and Urology Devices Market: Opportunities
R&D in nephrology and urology is expected to offer lucrative growth opportunities for players in the global nephrology and urology devices market. For instance, in August 2019, researchers at the Mayo Clinic reported development of a legitimate penile traction therapy (PTT) device for men with Peyronie’s disease that may work when used for just 30 to 90 minutes daily.
Moreover, increasing geriatric population is also expected to boost demand for nephrology and urology devices. For instance, according to the U.S. Census Bureau, the U.S. geriatric population is expected to reach 77 million by 2034.
The global nephrology and urology devices market was valued at US$ 21,796.2 Mn in 2019 and is forecast to reach a value of US$ 35,823.0 Mn by 2027 at a CAGR of 6.4% between 2020 and 2027.
Figure 2. Global Nephrology and Urology Devices Market Value (US$ Mn), and Y-o-Y Growth (%), 2019-2027
To learn more about this report, Download Free Sample
Market Trends/Key Takeaways
Major players in the market are focused on offering digital solutions during the Covid-19 pandemic. For instance, in July 2020, Baxter International expanded its partnership with Ayogo Health Inc. to combine the latter’s LifePlan behavior-based digital platform with Baxter’s renal care expertise to build mobile apps and digital solutions to support kidney failure patients.
Government initiatives to boost R&D in nephrology and urology devices is expected to propel growth of the global nephrology and urology devices market. For instance, in July 2020, the U.S. Department of Health and Human Services (HHS) and the American Society of Nephrology (ASN) announced the six winners of the US$ 3 million KidneyX: Redesign Dialysis Phase 2 competition during the virtual KidneyX Summit.
Global Nephrology and Urology Devices Market: Competitive Landscape
Major players operating in the global nephrology and urology devices market include, Fresenius Medical Care, Baxter International Inc., Asahi Kasei Corp., BD, Cook Group Incorporated, Dornier MedTech, Nikkiso Co. Ltd., NxStage Medical Inc., Nipro Corporation, Terumo Corporation, B Braun Group, Medtronic Plc, Awak Technologies, and Rockwell Medical Technologies, Inc.
Global Nephrology and Urology Devices Market: Key Developments
Major players in the global nephrology and urology devices market are focused on approval and launch of new devices to expand their product portfolio. For instance, in August 2020, Medtronic plc, received the U.S. FDA approval for its InterStim Micro neurostimulator and InterStim SureScan MRI leads for the treatment of patients with bladder and bowl control conditions.
Major players in the market are also focused on launching various solutions to enhance their market share. For instance, in August 2020, Dornier MedTech, a developer of non-invasive Extracorporeal Shockwave Lithotripsy (ESWL) technology, launched UroX, a community that connects professionals from various disciplines to cultivate innovation and solve the most pressing challenges in urology.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients