Near Patient Molecular Solution Market Size and Forecast – 2026 – 2033
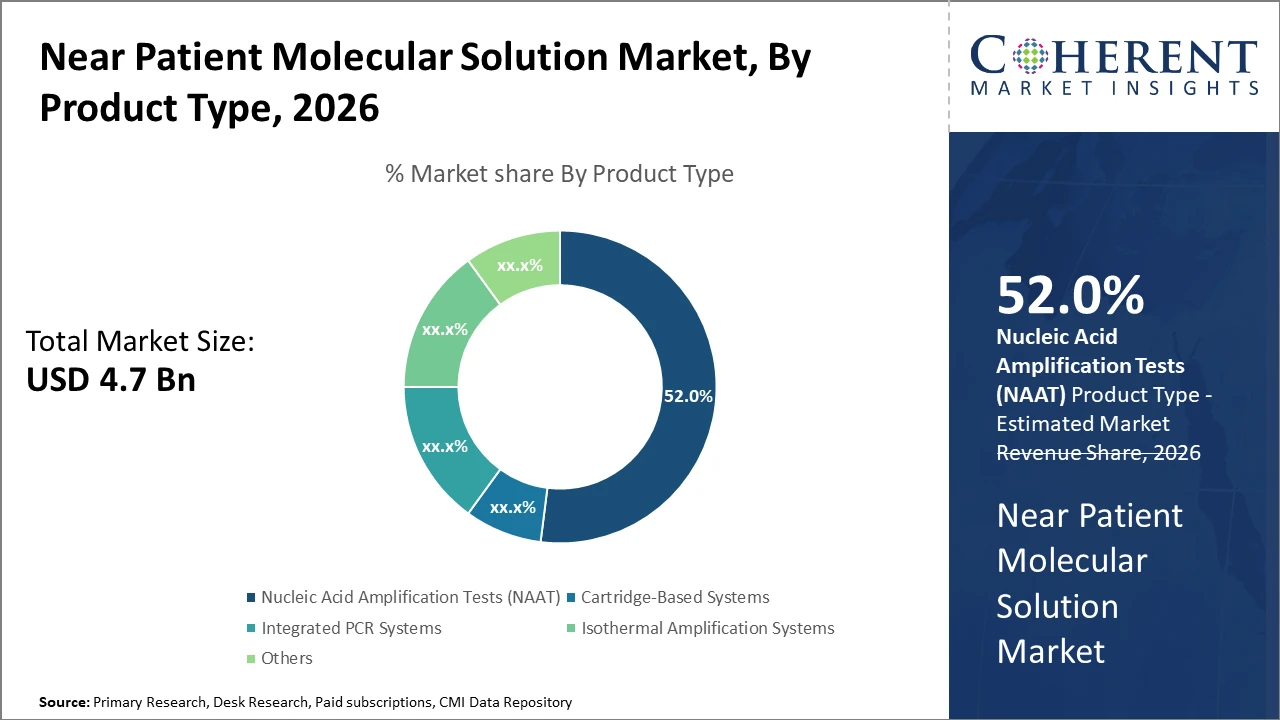
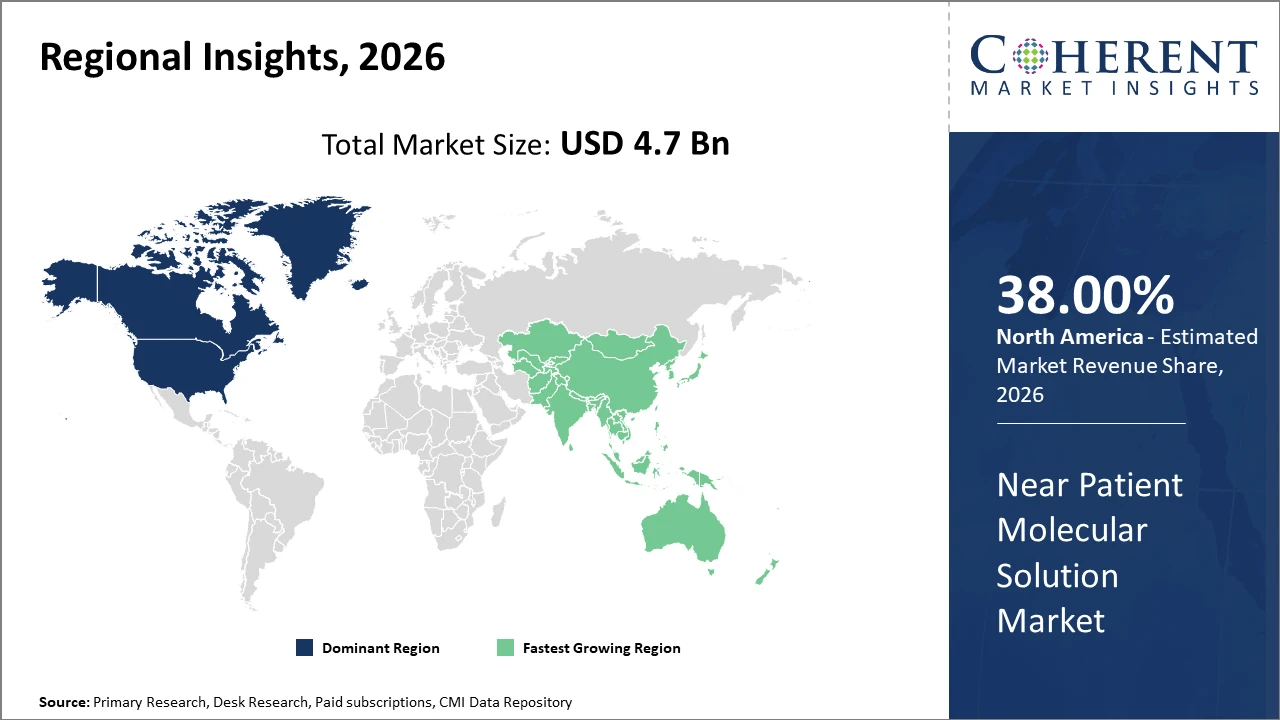
The Global Near Patient Molecular Solution Market size is estimated to be valued at USD 4.7 billion in 2026 and is expected to reach USD 9.8 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 11.2% from 2026 to 2033.
Global Near Patient Molecular Solution Market Overview
Near-patient molecular solutions are compact diagnostic systems that perform molecular testing close to the point of care rather than in centralized laboratories. These products integrate sample preparation, amplification, and detection into user-friendly platforms capable of delivering rapid and accurate results. They are used in hospitals, clinics, emergency departments, and decentralized settings for infectious disease testing, genetic screening, and oncology diagnostics. The solutions support faster clinical decision-making and improved patient management.
Key Takeaways
In the product segment, nucleic acid amplification tests dominate with over 52% market share due to their reliability and rapid turnaround times.
Infectious disease applications continue to drive the market’s largest revenue share, buoyed by increasing global disease outbreaks that necessitate near-patient diagnostics.
Genetic disorder applications show promising growth backed by advancements in personalized medicine.
North America leads the market in share, driven by early adoption and strong healthcare infrastructure, accounting for nearly 38% market share in 2026.
Asia Pacific presents the highest CAGR, projected at 13.5%, owing to expanding healthcare access and increasing investments in molecular diagnostics in countries like China and India.
Near Patient Molecular Solution Market Segmentation Analysis
To learn more about this report, Download Free Sample
Near Patient Molecular Solution Market Insights, By Product Type
Nucleic acid amplification tests dominate the market share with over 52% due to their high sensitivity and specificity, making them the preferred choice across various clinical applications. NAAT provides rapid and reliable results essential for infectious disease and genetic testing, thus commanding a significant portion of industry share. Cartridge-based systems are the fastest-growing subsegment, benefiting from their user-friendly interfaces and turnkey testing capabilities that reduce operator error and turnaround time. These systems have witnessed increased uptake in emergency care and outpatient settings. Integrated PCR systems hold a substantial market share by offering multiplexing functionalities with streamlined workflows.
Near Patient Molecular Solution Market Insights, By Application
Infectious diseases constitute the largest application segment, driven by persistent viral outbreaks and seasonal infections—this segment captures the majority of market revenue due to the critical need for rapid diagnostics in controlling disease spread. Oncology is the fastest-growing subsegment due to increasing integration of near-patient molecular assays in cancer biomarker identification and monitoring, reflecting broader trends towards personalized medicine. Genetic disorders also represent a crucial subsegment, propelled by advances in genomic profiling and the rising demand for early detection. Cardiovascular disease applications are emerging, focusing on identifying molecular markers to guide treatment.
Near Patient Molecular Solution Market Insights, By End-User
Hospitals dominate the market share owing to their extensive patient volume and growing adoption of rapid near-patient molecular testing to enhance clinical outcomes and operational efficiency. Emergency care centers are the fastest-growing subsegment, reflecting increased deployment of point-of-care molecular solutions for urgent diagnostic needs. Diagnostic laboratories remain key customers for multiplex and high-throughput testing platforms, while academic and research institutes drive product innovation through experimental applications.
Near Patient Molecular Solution Market Trends
The Near Patient Molecular Solution market is characterized by rapid technological evolution and expanding clinical applications.
A key trend involves the growing adoption of multiplex molecular assays enabling the simultaneous detection of multiple infectious agents, which substantially reduces diagnostic costs and enhances clinical workflow efficiency.
For instance, in 2025, multiplex platforms gained a 7% penetration rate increase across European hospitals.
Another developing trend is the amalgamation of molecular testing devices with telemedicine, allowing remote diagnostics and consultations, especially critical in the ongoing post-pandemic healthcare model.
Near Patient Molecular Solution Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Near Patient Molecular Solution Market Analysis and Trends
In North America, the dominance in the Near Patient Molecular Solution market originates from the presence of well-established healthcare infrastructure, supportive regulatory frameworks, and strong R&D activities by major market companies. The US, in particular, accounts for approximately 28% of the global market revenue share as of 2026. This region’s early adoption of novel point-of-care molecular diagnostic devices, coupled with aggressive reimbursement schemes and a large pool of end users, supports sustained market leadership.
Asia Pacific Near Patient Molecular Solution Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with a projected CAGR exceeding 13.5% through 2033. Factors such as rising prevalence of infectious diseases, increasing healthcare expenditure, and government initiatives in countries like China and India are propelling this expansion. Market players have increased manufacturing capacities and expanded distribution networks across the region, capitalizing on the significant unmet demand for near patient molecular diagnostic solutions.
Near Patient Molecular Solution Market Outlook for Key Countries
USA Near Patient Molecular Solution Market Analysis and Trends
The USA’s market is robust due to extensive adoption of molecular point-of-care devices across hospital networks and emergency care centers. Strategic investments by leading companies like Abbott Laboratories and Roche Diagnostics have significantly influenced market expansion. The U.S. FDA’s favorable regulatory environment and expanded insurance coverage for molecular testing have led to a 25% increase in near patient testing utilization in 2025. Additionally, numerous hospital systems in the US integrated molecular diagnostics into workflow models to reduce patient management costs and improve clinical outcomes.
India Near Patient Molecular Solution Market Analysis and Trends
India’s Near Patient Molecular Solution market is growing rapidly, fueled by increasing infectious disease burden and government healthcare initiatives like Ayushman Bharat that emphasize accessible diagnostics. Local manufacturing and partnerships between multinational and domestic companies have resulted in increased device affordability and distribution reach. In 2024, molecular diagnostic testing volume in India surged by 30%, driven by private and public healthcare providers expanding point-of-care services in Tier 2 and Tier 3 cities.
Analyst Opinion
Supply-side capacity expansion continues to be a major market driver. Production capacity for nucleic acid amplification devices grew by nearly 27% in 2024, driven by increased manufacturing in the Asia Pacific and North America. Greater automation and innovation in device throughput streamlined output, reducing production costs by 12%, which increased market penetration.
Demand-side dynamics highlight diversified use cases across infectious disease testing, oncology, and genetic screening. In 2025, the infectious disease segment accounted for over 45% of the overall near-patient molecular solution market revenue globally, spurred by real-time influenza and COVID-19 molecular diagnostics in outpatient settings and pharmacies. This wider adoption enhances market share substantially.
Pricing optimization and reimbursement policies remain micro-indicators impacting adoption rates. Recent trends in the US saw private payers expanding coverage for molecular point-of-care tests, raising utilization rates by 25% in 2024 alone. Competitive pricing strategies by key market players also lowered barriers for smaller clinics aspiring for advanced molecular diagnostics.
Nano-level data on hospital accreditation shows that institutions acquiring point-of-care molecular testing devices reduced patient turnaround time (TAT) by 30% on average in 2025. This improvement not only accelerates clinical decision-making but is fueling demand further in regions with high patient load.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 4.7 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 11.2% | 2033 Value Projection: | USD 9.8 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Thermo Fisher Scientific Inc., Cepheid (Danaher Corporation), Hologic, Inc., Sysmex Corporation, QIAGEN GmbH, Meridian Bioscience, Inc., PerkinElmer, Inc., Siemens Healthineers, BD (Becton, Dickinson and Company), GenMark Diagnostics. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Near Patient Molecular Solution Market Growth Factors
The accelerating need for rapid diagnostic solutions is a primary driver, with molecular point-of-care testing minimizing delays in treatment decisions. For instance, the increasing prevalence of infectious diseases in emerging economies has created a surge in demand, accounting for nearly 40% growth in the Asia Pacific in 2025. Regulatory approvals for portable molecular diagnostic devices and streamlined government policies have also reduced market barriers. Moreover, advancements in microfluidics and biosensor technologies have enhanced testing accuracy and ease-of-use, as evidenced by a 15% reduction in false-negative rates reported by several hospitals adopting near-patient molecular solutions in 2026. Furthermore, growing collaborations between healthcare providers and technology firms serve as catalysts for innovation and adoption, reinforcing market dynamics favoring expansion.
Near-Patient Molecular Solution Market Development
In June 2025, Thermo Fisher Scientific launched new oncology-focused next-generation sequencing (NGS) panels, designed to support comprehensive genomic profiling for cancer research and clinical translational workflows. The panels expand mutation, fusion, and biomarker coverage, enabling more precise oncology decision-making and accelerating precision medicine adoption.
Key Players
Leading Companies of the Market
Thermo Fisher Scientific Inc.
Cepheid (Danaher Corporation)
Hologic, Inc.
Sysmex Corporation
QIAGEN GmbH
Meridian Bioscience, Inc.
PerkinElmer, Inc.
Siemens Healthineers
BD (Becton, Dickinson and Company)
GenMark Diagnostics
Many leading companies have strategically expanded their product portfolios through targeted acquisitions and innovation. For example, Roche Diagnostics’ integration of compact real-time PCR platforms in 2024 boosted its near patient molecular testing footprint by 18%, especially in emergency care settings. Abbott Laboratories enhanced its market presence in the infectious disease segment by expanding its cartridge-based molecular test menu, which led to a 22% increase in revenues in 2025, driven by adoption in outpatient clinics.
Near Patient Molecular Solution Market Future Outlook
The future outlook for near-patient molecular solutions is strongly aligned with the decentralization of healthcare delivery. Continued innovation is expected to focus on expanding test menus, reducing costs, and improving interoperability with digital health systems. The growing emphasis on early diagnosis, antimicrobial stewardship, and personalized treatment decisions will further drive adoption. As healthcare providers seek faster and more actionable diagnostic insights, near-patient molecular solutions are expected to become an integral component of routine clinical workflows across diverse care settings.
Near Patient Molecular Solution Market Historical Analysis
The Near Patient Molecular Solution Market emerged as a response to the limitations of centralized laboratory testing, particularly long turnaround times and logistical constraints. Early molecular diagnostics were complex, costly, and restricted to specialized laboratories with skilled personnel. The introduction of compact, integrated molecular platforms marked a significant shift, enabling sample-to-result testing closer to the patient. Initial adoption was driven by infectious disease diagnostics in hospitals and emergency settings, where rapid decision-making was critical. Over time, improvements in assay automation, cartridge-based systems, and digital integration expanded near-patient molecular testing into broader clinical applications.
Sources
Primary Research Interviews:
Pathologists
diagnostic lab managers
molecular biologists
hospital administrators
IVD manufacturers
Databases:
WHO Diagnostics Data
CDC Laboratory Data
FDA IVD Database
OECD Health Statistics
Magazines:
Diagnostics World
GenomeWeb
Medical Device Network
Clinical Lab Products
MedTech Dive
Journals:
Clinical Chemistry
Journal of Molecular Diagnostics
PLOS ONE
Nature Diagnostics
Journal of Clinical Microbiology
Newspapers:
Reuters Health
Financial Times (Healthcare)
The Guardian (Science)
Bloomberg Diagnostics
The New York Times (Health)
Associations:
Association for Molecular Pathology
Clinical & Laboratory Standards Institute
WHO
American Society for Clinical Pathology
IFCC
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients