Nanoscale Virus Trap Molecule Market Size and Forecast – 2026 – 2033
The Global Nanoscale Virus Trap Molecule Market size is estimated to be valued at USD 450 million in 2026 and is expected to reach USD 1,220 million by 2033, exhibiting a compound annual growth rate (CAGR) of 15.2% from 2026 to 2033.
Global Nanoscale Virus Trap Molecule Market Overview
Nanoscale virus trap molecules are engineered nanostructures designed to capture, neutralize, or inhibit viruses before they infect host cells. These products mimic natural binding sites or use functionalized surfaces to physically trap viral particles at the nanoscale level. They are applied in antiviral therapies, infection prevention coatings, diagnostic platforms, and protective medical devices. The technology aims to reduce viral load and transmission without relying solely on traditional antiviral drugs.
Key Takeaways
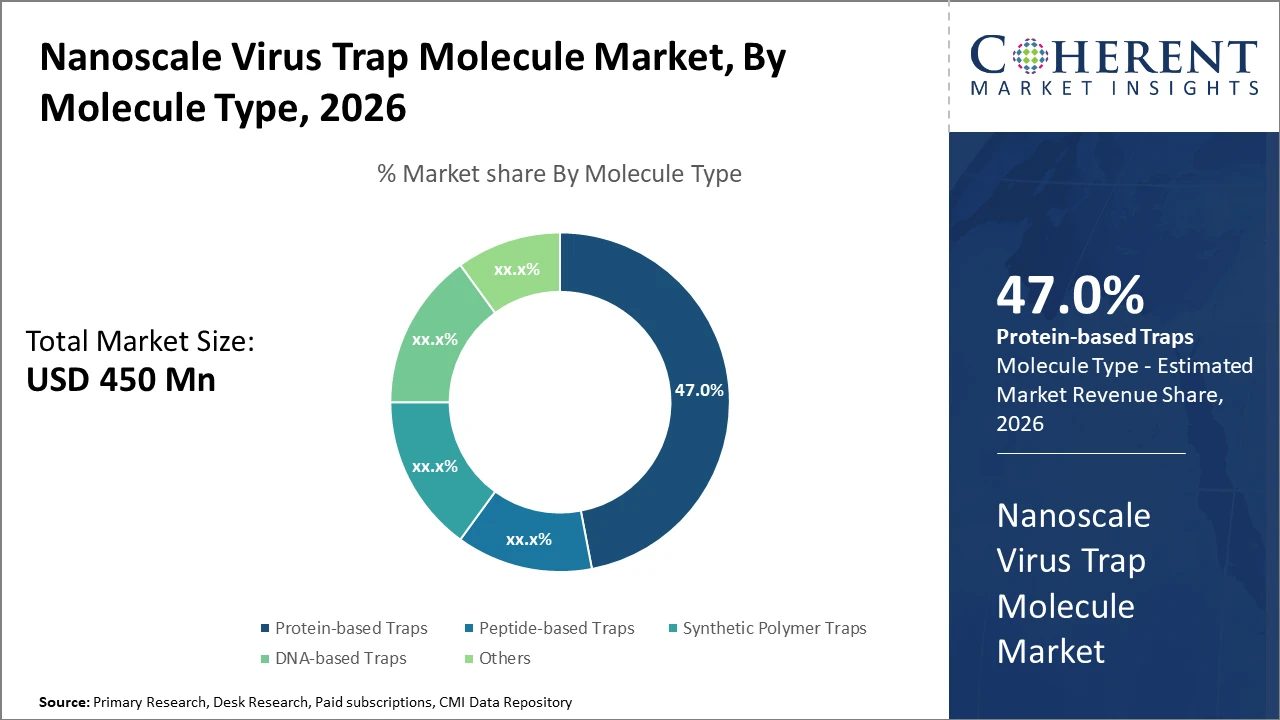
The protein-based trap molecule segment dominates, accounting for nearly 47% market share, driven by its superior binding affinity and therapeutic efficacy. Peptide-based traps are emerging rapidly due to their customizable structures and cost-efficient synthesis.
Therapeutics remains the largest application area, benefiting from accelerating viral pandemics and advanced drug delivery mechanisms, whereas diagnostics exhibit faster innovation adoption.
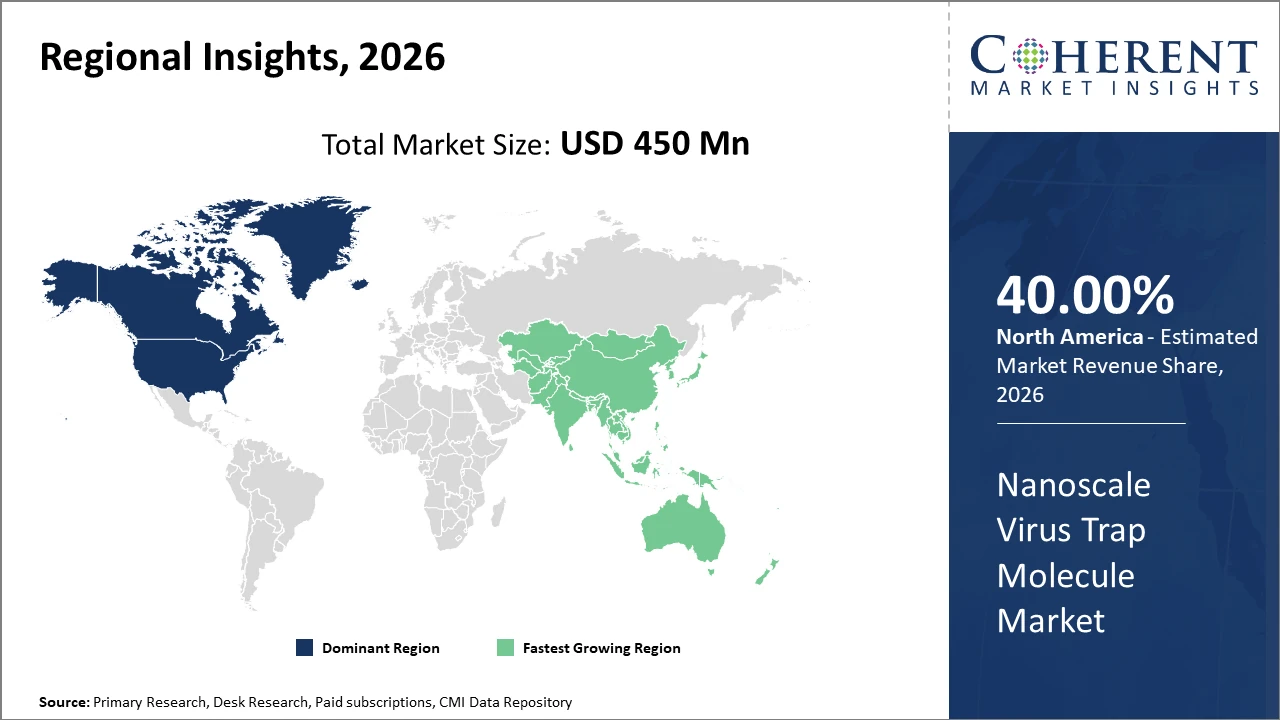
North America commands the largest market share with strong pharmaceutical infrastructure and research funding, notably led by the U.S. Meanwhile, Asia Pacific is the fastest-growing region owing to expanding healthcare investments and rising virus containment initiatives.
Europe shows increasing traction due to heightened regulatory support for nanomedicine, while the Middle East & Africa region draws attention for environmental application growth.
Nanoscale Virus Trap Molecule Market Segmentation Analysis
To learn more about this report, Download Free Sample
Nanoscale Virus Trap Molecule Market Insights, By Molecule Type
Protein-based Traps dominate the market share. Protein-based Traps are favored for their high specificity in binding viral particles, making them invaluable in therapeutic applications. The segment benefits from enhanced bioengineering techniques that improve binding affinity and stability, accelerating adoption in clinical and environmental settings. Peptide-based Traps are the fastest-growing subsegment, driven by their modular structure, which allows rapid customization and cost-effective synthesis processes suitable for diverse virus targets. Synthetic Polymer Traps represent a promising area, offering scalable manufacturing option,s but currently occupy a smaller market footprint. DNA-based Traps, though emerging, show potential due to their programmable nature, but face technical barriers.
Nanoscale Virus Trap Molecule Market Insights, By Application
Therapeutics are dominating due to their broad utilization in antiviral drug enhancement and novel therapies. Utilization in therapeutics benefits from advancements in targeted delivery and intracellular activity of nanoscale traps, particularly evident in pandemic response frameworks. Diagnostics, while smaller in size, are growing swiftly by incorporating nanoscale traps into virus detection and isolation assays, improving sensitivity. Environmental controls leverage nanoscale virus traps for air and water purification, expanding market revenue through non-clinical applications. Vaccine development employs these molecules primarily as adjuvants or virus neutralizers, gaining traction but presently contributing moderate revenue.
Nanoscale Virus Trap Molecule Market Insights, By End-User
Pharmaceutical Companies command the largest share by deploying nanoscale virus-trapping molecules into drug pipelines and therapeutic solutions, supported by increasing R&D investment to accelerate antiviral innovations. Research Institutions are rapidly expanding as the fastest-growing group due to their role in developmental research, application experiments, and novel molecule discovery. Healthcare Providers mainly adopt therapeutic products integrating nanoscale traps, whereas Industrial Applications are emerging markets covering environmental and biosafety uses.
Nanoscale Virus Trap Molecule Market Trends
Recent shifts include the rise of multifunctional trap molecules capable of simultaneous virus capture and neutralization, creating new therapeutic paradigms.
For instance, in 2025, several biotech startups introduced dual-action nanoscale traps integrated with antiviral peptides, pushing market innovation frontiers.
Additionally, the movement toward miniaturized delivery systems facilitates targeted intracellular virus interception, improving therapeutic outcomes.
Another trend is the growing emphasis on environmentally sustainable synthesis of nanoscale traps, driven by stringent regulatory frameworks established across Europe and North America since late 2023. This eco-conscious pivot triggers a market reshuffle favoring green nanotechnology suppliers.
Nanoscale Virus Trap Molecule Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Nanoscale Virus Trap Molecule Market Analysis and Trends
In North America, the dominance in the Nanoscale Virus Trap Molecule market is underpinned by robust R&D funding combined with an extensive network of pharmaceutical and biotech companies. The U.S., in particular, accounts for over 40% of the market share, enabled by advanced healthcare infrastructure and active government initiatives supporting nanomedicine innovation.
Asia Pacific Nanoscale Virus Trap Molecule Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with a CAGR exceeding 18%. The rapid rise is primarily driven by expanding healthcare expenditure, government-led virus containment programs, and increasing collaborations between local biotech startups and international partners, especially in China and India.
Nanoscale Virus Trap Molecule Market Outlook for Key Countries
USA Nanoscale Virus Trap Molecule Market Analysis and Trends
The U.S. market continues to lead due to extensive investments in nanotechnology and viral research. Companies like NanoViroTech Inc. and ViruTrap Solutions spearhead innovation, backed by significant funding from federal programs targeting pandemic preparedness. The rise in biotech incubators and advanced manufacturing facilities ensures sustained market momentum, contributing to a healthy competitive ecosystem.
China Nanoscale Virus Trap Molecule Market Analysis and Trends
China’s market benefits from stringent government policies promoting nanobiotechnology and local manufacturing capabilities. Growing collaborations between domestic research institutes and multinational corporations fuel product development. Noteworthy players such as NanoGuard Therapeutics have expanded pilot production capabilities in key provinces, driving the market’s fast-paced growth trajectory.
Analyst Opinion
The surge in demand for targeted antiviral therapies is a critical driver of market growth. For instance, in 2024, the deployment of nanoscale trapping molecules in combatting novel virus strains saw a 28% increase in adoption across clinical trials worldwide, reflecting increased emphasis on precision medicine. This dynamic expands market share by enhancing the scope of applications beyond existing antiviral drugs.
Production capacity constraints for nanoscale molecules persist as a supply-side challenge; however, recent innovations in automated nanofabrication methods increased output by approximately 22% year-on-year as of 2025, enabling larger-scale commercial manufacturing. This production boost is integral to meeting the swelling demand from pharmaceutical companies and research labs.
Pricing strategies remain pivotal in market penetration. Data from 2024 show that cost reductions of 12% in nanoscale molecule synthesis directly correlated with a 15% hike in procurement by emerging biotech firms focused on infectious disease control, indicating elasticity in market revenue based on pricing dynamics.
The expanding use cases across biomedical industries—ranging from vaccine enhancement to environmental virus interception—contributes to diversified demand. For example, environmental health agencies reported a 20% increase in trials involving nanoscale virus traps for air filtration application in 2025, reflecting growing cross-sector adoption which underpins broader market growth.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 450 million |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 15.2% | 2033 Value Projection: | USD 1.22 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Pfizer Inc., Merck & Co., Inc., Novartis AG, Janssen Pharmaceuticals, Inc. (Johnson & Johnson), Oxford Nanopore Technologies, Cytiva (Danaher Corporation), Bruker Corporation, Nanospectra Biosciences, Inc., Thermo Fisher Scientific Inc., and Jazz Pharmaceuticals plc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Nanoscale Virus Trap Molecule Market Growth Factors
The market growth is primarily propelled by rising investment in nanomedicine and the urgent need for innovative antiviral therapies. Enhanced government funding for infectious disease research globally fuels R&D activities, thereby expanding the market scope. Another key factor is the escalating prevalence of viral outbreaks, which elevates demand for rapid-response nanoscale virus trapping solutions in pharmaceutical development. Additionally, the integration of nanoscale virus traps in environmental health applications, such as air and water purification, stimulates additional revenue streams and market diversification. Technology advancements fostering cost-effective production and increased molecule stability are expected to sustain business growth further.
Nanoscale Virus Trap Molecule Market Development
In October 2022, Ceres Nanosciences launched Nanotrap® Microbiome Particles, magnetic hydrogel affinity‑capture particles designed to capture and concentrate low‑abundance microbes such as SARS‑CoV‑2, influenza, and RSV from complex samples, improving wastewater surveillance and clinical pathogen detection sensitivity.
In December 2021, Vitex launched its VAIRO antiviral coating, a silver‑ion‑based nanotechnology surface treatment shown to create a “trap and kill” environment for pathogens and achieve up to 99% effectiveness against human coronaviruses. The coating has been deployed in high‑traffic settings such as hospitals and schools to bolster infection control.
Key Players
Some of the leading companies in the market include:
Pfizer Inc.
Merck & Co., Inc.
Novartis AG
Janssen Pharmaceuticals, Inc. (Johnson & Johnson)
Oxford Nanopore Technologies
Cytiva (Danaher Corporation)
Bruker Corporation
Nanospectra Biosciences, Inc.
Thermo Fisher Scientific Inc.
Jazz Pharmaceuticals plc
These players contribute through lipid nanoparticles, nanocarriers, and nano-enabled drug delivery platforms used for viral neutralization and targeted therapy. Diagnostic leaders like Oxford Nanopore and Thermo Fisher support ultra-fast viral detection using nanoscale sensing technologies. Equipment and tooling providers such as Bruker and Imina enable nanoscale imaging, manipulation, and characterization essential for virus-trap molecule development. Collectively, these companies strengthen the market by advancing nanomedicine, antiviral research, scalable nanoparticle manufacturing, and next-generation virus detection solutions.
Nanoscale Virus Trap Molecule Market Future Outlook
The Nanoscale Virus Trap Molecule Market is expected to evolve as a complementary approach to traditional antiviral therapies and vaccines. Future developments are likely to focus on improving specificity across multiple virus strains, enhancing durability, and integrating virus-trapping molecules into medical devices, air filtration systems, and diagnostic platforms. Advances in scalable nanomanufacturing and regulatory frameworks for nanomaterials will play a critical role in commercial adoption. As global health systems prioritize pandemic preparedness and non-pharmaceutical infection control strategies, nanoscale virus trap technologies are expected to gain broader acceptance across medical, industrial, and public health environments.
Nanoscale Virus Trap Molecule Market Historical Analysis
The Nanoscale Virus Trap Molecule Market originated from interdisciplinary research combining nanotechnology, virology, and materials science, particularly during periods of heightened concern over viral outbreaks. Early development focused on understanding virus–surface interactions and designing nanoscale structures capable of binding viral particles selectively. Initially, these technologies were limited to laboratory research and experimental antiviral coatings, with limited translational applications due to manufacturing complexity and scalability challenges. Over time, advancements in nanofabrication, surface functionalization, and biomimetic engineering enabled more consistent and reproducible virus-trapping structures. Increased global emphasis on infection prevention, especially following major viral epidemics, accelerated research funding and early-stage commercialization across healthcare, diagnostics, and protective equipment applications.
Sources
Primary Research Interviews:
Nanotechnology researchers
Virologists
Biomedical engineers
Material scientists
Infectious disease experts
Databases:
NIH Nanomedicine Data
WHO Infectious Disease Data
PubChem
ClinicalTrials.gov
Magazines:
Nanowerk
Nature Nanotechnology News
Science Translational Medicine
Medical Device Network
BioWorld
Journals:
Nature Nanotechnology
ACS Nano
Advanced Materials
Nano Letters
Journal of Virology
Newspapers:
The New York Times (Science)
Reuters Health
Financial Times (Technology)
The Guardian (Science)
Bloomberg Innovation
Associations:
American Chemical Society
International Society for Nanomedicine
WHO, Materials Research Society
IEEE Nanotechnology Council
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients