Nanotechnology is a common term for nanoscale science, engineering and technology, which offers extraordinary economic and technological benefits. Healthcare is one of the most prominent field of research in nanotechnology. Nanomedicine can be understood as a field of diagnosing, treating and preventing disease using molecular tools and knowledge of the human body.
Statistics:
The global nanomedicine market is estimated to account for US$ 454.8 Bn in terms of value by the end of 2027.
Global Nanomedicine Market: Drivers
High prevalence of chronic diseases is expected to propel growth of the global nanomedicine market over the forecast period. For instance, according to the American Cancer Society, in 2019, there will be an estimated 1,762,450 new cancer cases diagnosed and 606,880 cancer deaths in the U.S.
Moreover, opening of a NanoTherm treatment centers is also expected to aid in growth of the market. For instance, in March 2020, MagForce AG and the Hufeland Klinikum GmbH announced the conclusion of a joint cooperation agreement and the planned opening of a NanoTherm treatment center for brain tumors at the Mühlhausen site in Thuringia, Germany.
Statistics:
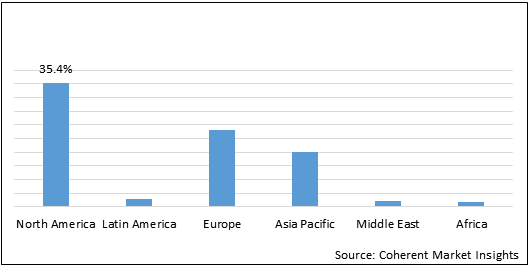
North America held dominant position in the global nanomedicines market in 2019, accounting for 35.4% share in terms of volume, followed by Europe and Asia Pacific, respectively.
Figure 1: Global Nanomedicine Market Share (%) Value, By Region, 2019
To learn more about this report, Download Free Sample
Global Nanomedicine Market: Restraints
Lack of organized regulatory framework is expected to hinder growth of the global nanomedicine market. There are presently no specific testing requirements for nanomedicine. However, the safety testing requirements of the FDA, CDER and other regulatory authorities worldwide for any medical products is very stringent. Nanomedicine products are associated with environmental and toxicological issues, which make it mandatory for manufacturers to perform toxic studies and abide with environmental regulations while manufacturing. Current preclinical tests for safety evaluation include tests such as toxicology, pharmacology, ADME, carcinogenicity, immunotoxicity and genotoxicity.
Moreover, low adoption of nanomedicine in emerging economies is also expected to limit the market growth.
Nanomedicine Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2019 | Market Size in 2019: | US$ 177.1 Bn |
| Historical Data for: | 2016 to 2019 | Forecast Period: | 2020 to 2027 |
| Forecast Period 2020 to 2027 CAGR: | 12.5% | 2027 Value Projection: | US$ 454.8 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Abbott Laboratories, CombiMatrix Corporation, Clene Nanomedicine, Inc., Cellics Therapeutics, Inc., Nanobiotix S.A, Celgene Corporation, GE Healthcare, NanoViricides, Inc., Johnson & Johnson, Mallinckrodt plc., Sirnaomics Inc., Precision NanoSystems Inc., Merck & Company Inc., Nanosphere, Inc., Pfizer Inc., and Sigma-Tau Pharmaceuticals Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Nanomedicine Market: Opportunities
Presence of unmet medical needs in emerging economies is expected to offer lucrative growth opportunities for players in the global nanomedicine market. Emerging and high growth markets such as China, Brazil, India, Eastern Europe, Middle East, and Latin America are expected to offer a vast arena of opportunities for the nanomedicine market in future. This is due to the fact that economic development in these economies has equipped patients with purchasing power induced by rising disposable incomes. Improvement of healthcare infrastructure will enable use of sophisticated technologies and cater to the high unmet medical needs of the population.
Moreover, R&D in nanomedicine is also expected to aid in growth of the market. For instance, in August 2020, researchers at the Else Kröner-Fresenius-Stiftung Professorship, Universitätsklinikum Erlangen, Germany, reported that superparamagnetic iron oxide nanoparticles can function as an effective delivery platform to increase local drug concentrations, thereby potentially overcoming chemotherapy resistance of cells.
Statistics:
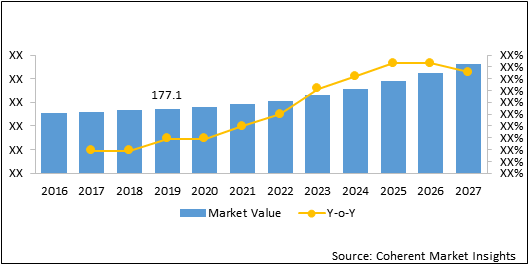
The global nanomedicine market was valued at US$ 177.1 Bn in 2019 and is forecast to reach a value of US$ 454.8 Bn by 2027 at a CAGR of 12.5% between 2020 and 2027.
Figure 2: Global Nanomedicine Market Value (US$ Bn), 2016 – 2027
To learn more about this report, Download Free Sample
Market Trends/Key Takeaways
Major players in the global nanomedicine market are focused on R&D of new products to expand their product portfolio. For instance, in August 2020, Clene Nanomedicine, Inc. announced that the Sean M Healey & AMG Center for ALS at Mass General enrolled the first patients into the HEALEY ALS Platform Trial being conducted at sites of the Northeast ALS (NEALS) consortium. The company’s CNM-Au8, a therapeutic nanocatalyst was selected as one of the first drug regimens to be evaluated as an ALS treatment in the HEALEY ALS Platform Trial.
Investment in nanomedicine is expected to propel growth of the global nanomedicine market. For instance, in July 2020, Curadigm SAS, a wholly-owned subsidiary of Nanobiotix S.A., announced the receipt of US$ 1.18 million in non-dilutive funding from Deep Tech BPI France for the development of their Nanoprimer technology.
Global Nanomedicine Market: Competitive Landscape
Major players operating in the global nanomedicine market include, Abbott Laboratories, CombiMatrix Corporation, Clene Nanomedicine, Inc., Cellics Therapeutics, Inc., Nanobiotix S.A, Celgene Corporation, GE Healthcare, NanoViricides, Inc., Johnson & Johnson, Mallinckrodt plc., Sirnaomics Inc., Precision NanoSystems Inc., Merck & Company Inc., Nanosphere, Inc., Pfizer Inc., and Sigma-Tau Pharmaceuticals Inc.
Global Nanomedicine Market: Key Developments
Major players in the global nanomedicine market are focused on R&D of new products to expand their product portfolio. For instance, in June 2020, Nanobiotix S.A announced that the FDA has provided necessary feedback regarding the design of NANORAY-312, the company’s pivotal phase III global registration trial in head and neck cancer.
Similarly, in June 2020, Cellics Therapeutics, Inc. announced that results of the study that evaluates the potential benefits of macrophage and pulmonary epithelial nanosponges in neutralizing SARS-CoV-2 infectivity have been published in Nano Letters.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients