Multiplexed diagnostics are used for analysis of biological samples, which are capable of completing a screening process with a single run. Multiplexed diagnostic assays are involved in the detection and diagnosis of different diseases and infections, which include cancer, autoimmune diseases, allergies, and cardiovascular diseases. Assays performed in multiplex diagnostics include high density multiplexed assays, medium density multiplexed assays, low density multiplexed assays, and next-generation sequencing assays. Early diagnosis can minimize the risk factors associated with chronic diseases/infection and helps in proper treatment management. Moreover, multiplexed diagnostic assays have advantages such as time efficiency and high accuracy and precision, which can analyze defects and mutations in any functional biomolecules such as DNA, RNA, and protein.
The global multiplexed diagnostic market is expected to be valued at US$ 10,126.6 million in 2019 and is expected to exhibit a CAGR of 7.9% over the forecast period (2019-2027).
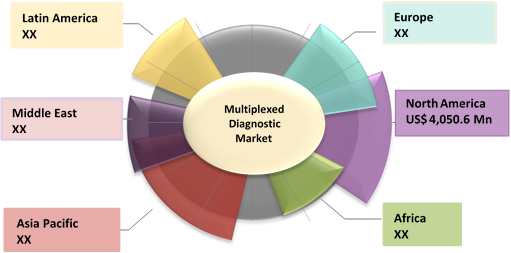
Figure 1. Global Multiplexed Diagnostic Market Value (US$ Mn), by Region, 2019
To learn more about this report, Download Free Sample
Source: Coherent Market Insights Analysis (2020)
Increasing incidence of disease outbreaks is expected to propel the market growth over the forecast period.
The increasing incidence of disease outbreaks such as coronavirus and Ebola virus is escalating demand for advance diagnostic measures, which is further expected to boost growth of the multiplexed diagnostic market. For instance, the coronavirus disease is the most recent outbreak, which was first reported on December 31, 2019 in Wuhan, China. According to the World Health Organization (WHO), globally 87,137 cases of the coronavirus disease were reported on 1st March 2020.
Moreover, on August 1, 2018, the Democratic Republic of the Congo Ministry of Health (DRC MoH) declared Ebola outbreak in the North Kivu province of Congo. Moreover, according to the World Health Organization (WHO), total 3,444 confirmed cases of the Ebola infection were reported on February 25, 2020, in the North Kivu province. Such incidence are expected to drive demand for high performance diagnostics, in order to prevent the false positive results of the tests and identify the pathogen accurately.
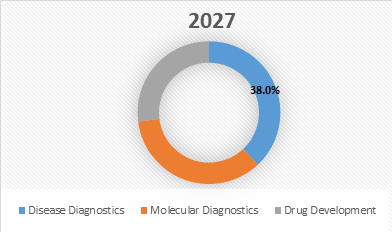
Figure 2. Global Multiplexed Diagnostic Market Share (%), by Application, 2027
To learn more about this report, Download Free Sample
Source: Coherent Market Insights Analysis (2020)
Increasing launches and approvals of new and advanced multiplexed diagnostic tools, is expected to fuel growth of the multiplexed diagnostic market.
Manufacturers are focusing on launches and approvals of innovative multiplexed diagnostics, in order to prevent false positive test results. For instance, in February 2020, Co-Diagnostics, Inc., received the CE mark for Logix Smart Coronavirus COVID-19 Test from the European Community. The Logix Smart Coronavirus COVID-19 Test kit is an in vitro diagnostic test, which operates using the real-time reverse transcriptase polymerase chain reaction (RT-PCR) process along with enhanced multiplexing for identifying the coronavirus.
Moreover, in May 2019, QIAGEN N.V. launched QIAstat-Dx syndromic testing system in the U.S. This is a multiplex molecular diagnostic system developed for the qualitative detection and identification of multiple respiratory bacterial and viral pathogens. QIAstat-Dx was launched along a comprehensive respiratory panel, which can detect over 20 pathogens.
However, limited availability of experienced and trained healthcare professionals for handling advanced multiplexed diagnostic equipment such as microarray analyzers and enzyme-linked immunosorbent assays are expected to hinder the market growth over the forecast period.
Key Players
Major players operating in the global multiplexed diagnostic market include Luminex Corporation, Thermo Fisher, Illumine Inc., Bio-Rad Laboratories, Inc., Qiagen N.V., Abbott Laboratories, Siemens Healthineers, Agilent technologies, BioMerieux SA, and F.Hoffmann-La Roche Ltd.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients