Monobody Based Therapeutic Drugs Market Size and Forecast – 2026 – 2033
The market size for Monobody Based Therapeutic Drugs is estimated to be valued at USD 1.45 billion in 2026 and is expected to reach USD 4.12 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 16.5% from 2026 to 2033.
Global Monobody Based Therapeutic Drugs Market Overview
Monobody-based therapeutic drugs are engineered protein molecules designed to bind selectively to disease-related targets such as receptors, enzymes, or signaling proteins. Unlike traditional antibodies, monobodies are smaller, more stable, and can be engineered for high specificity and affinity. These products are developed for applications in oncology, inflammatory diseases, and rare disorders, where precise molecular targeting is required. Their compact structure allows better tissue penetration and flexible delivery formats, including injectable and potentially intracellular therapeutic approaches.
Key Takeaways
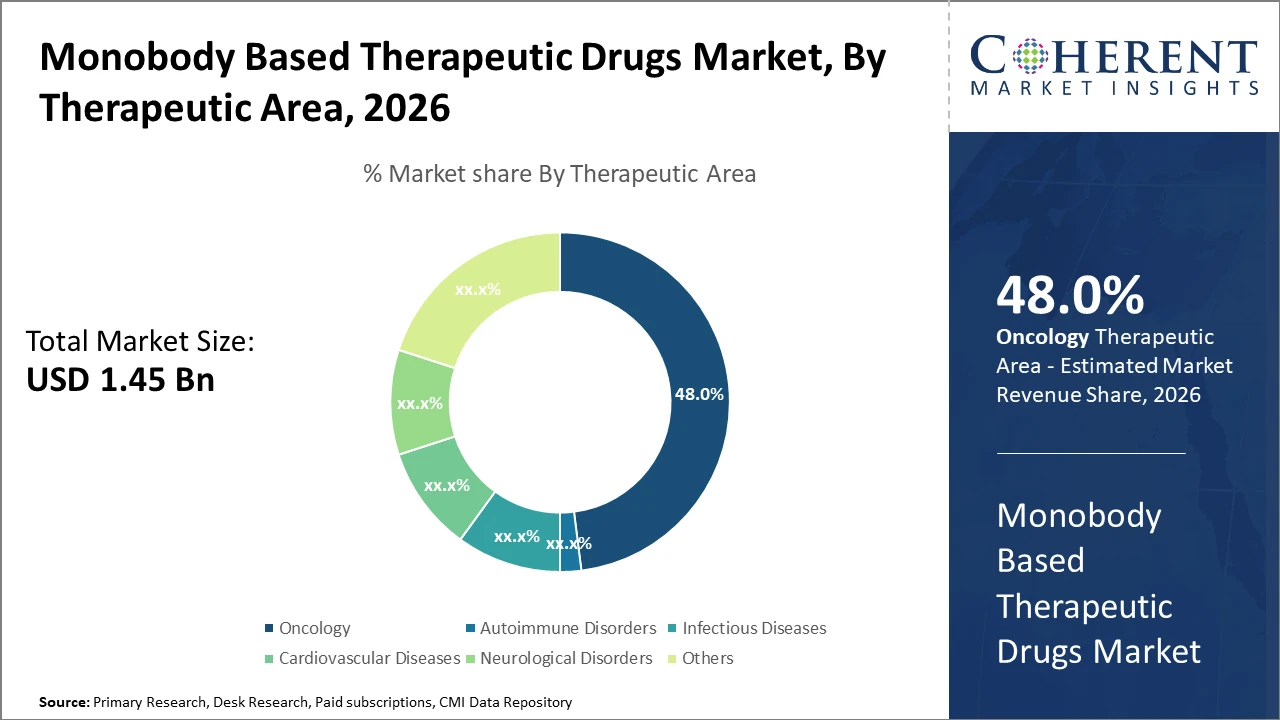
The oncology segment dominates the Monobody Based Therapeutic Drugs market share due to targeted therapies' success in clinical trials and adoption in mainstream cancer treatment protocols.
Among drug types, monobody conjugates are capturing a significant revenue share driven by their enhanced pharmacokinetics and multi-functionality.
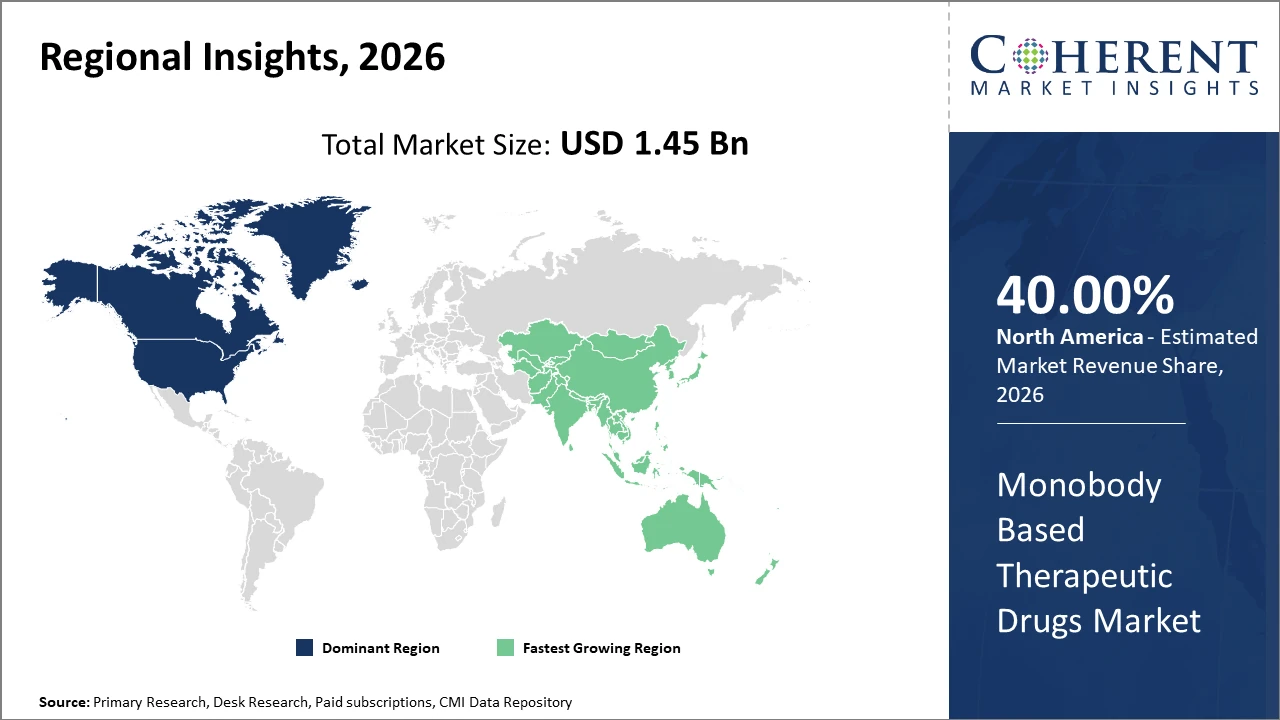
Regionally, North America leads the market, accounting for over 40% of the industry share due to a robust biopharmaceutical ecosystem and strong patent protection laws.
The Asia Pacific region exhibits the fastest growth, propelled by rising investments in biotechnology infrastructure and supportive government policies in countries like China and India.
Monobody Based Therapeutic Drugs Market Segmentation Analysis
To learn more about this report, Download Free Sample
Monobody Based Therapeutic Drugs Market Insights, By Therapeutic Area
Oncology dominates the market share. Oncology's leadership stems from its targeted therapy approach enabled by monobody drugs, which showed unprecedented clinical progression rates in 2025 with response rates exceeding standard biologics by over 15% in multiple cancers. Autoimmune Disorders stand out as the fastest-growing segment, fueled by a rising patient base and innovation in monobody immunomodulators, showing a year-over-year 22% growth in clinical adoption. Infectious Diseases, Cardiovascular Diseases, and Neurological Disorders maintain stable growth, focusing on specialized monobody applications with evolving pipeline activities.
Monobody Based Therapeutic Drugs Market Insights, By Drug Type
Monobody Conjugates holds the dominant share. Their dominance comes from enhanced pharmacodynamic properties and success in delivering cytotoxic agents precisely to tumor cells, significantly improving safety profiles. Monobody Fusion Proteins represent the fastest-growing segment, riding on the enhanced multifunctionality and half-life extension capabilities seen in recent biotherapeutic approvals. Monobody Antagonists and Agonists maintain niche applications targeting specific receptor pathways, while Others cover emerging formats like bispecific monobodies under development.
Monobody Based Therapeutic Drugs Market Insights, By Application
Therapeutic application predominates the market share due to the critical role of monobody drugs in disease treatment protocols and rising clinical successes. Within Therapeutics, cancer and autoimmune diseases dominate usage, with innovations driving uptake. Research Tools show the highest growth trajectory driven by increasing use of monobody scaffolds as affinity reagents and molecular probes in both academic and industrial R&D settings. Diagnostic applications leverage monobody specificity for biomarker detection, and Others comprise assorted applications including veterinary therapeutics and environmental biosensing.
Monobody Based Therapeutic Drugs Market Trends
The Monobody Based Therapeutic Drugs market trend highlights significant advances in protein design technology, allowing for the creation of monobody drugs with greater affinity and specificity compared to traditional antibody therapies.
For instance, in 2025, a major breakthrough involved a monobody therapeutic demonstrating superior efficacy in phase III oncology trials, reinforcing the market's shift toward monobody modalities.
Additionally, the trend toward integrating monobody drugs in combination therapies, such as pairing with immune checkpoint inhibitors, has broadened therapeutic avenues and boosted market penetration in the cancer and autoimmune diseases markets.
Another pivotal trend is the growth of thermostable monobody drugs, facilitating refrigerated supply chain independence, critical for expanding access in developing regions.
Monobody Based Therapeutic Drugs Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Monobody Based Therapeutic Drugs Market Analysis and Trends
In North America, the dominance in the Monobody-Based Therapeutic Drugs market is underpinned by a well-established biopharmaceutical infrastructure, a high number of clinical trials, and strong government funding for biotechnology R&D. The region accounts for over 40% market share, with the U.S. being home to multiple leading companies investing in innovative monobody platforms. Patent protections and streamlined regulatory processes have further solidified North America’s leadership.
Asia Pacific Monobody Based Therapeutic Drugs Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with a CAGR exceeding 18% currently, driven by expanding biotechnology hubs in China and India, increasing biopharma investments, and rising incidence of target diseases. Government initiatives aimed at enhancing R&D capabilities and supportive trade policies, bolstered by growing domestic pharmaceutical manufacturing, have propelled the region's market growth.
Monobody Based Therapeutic Drugs Market Outlook for Key Countries
USA Monobody Based Therapeutic Drugs Market Analysis and Trends
The USA’s market serves as a key driver globally, with numerous biotech startups and established pharma companies pioneering monobody drug development. The robust clinical trial ecosystem saw over 70 ongoing monobody-based investigational studies in 2024 alone. Furthermore, large-scale collaborations between academia and industry accelerated pipeline progression, supported by favorable FDA regulatory frameworks. Leading companies have capitalized on innovative protein engineering technologies, driving the market’s significant revenue contribution and maintaining the country’s status as the innovation epicenter.
China Monobody Based Therapeutic Drugs Market Analysis and Trends
China’s monobody drug market is rapidly expanding, propelled by increased governmental funding for biotech innovation and rising prevalence of chronic diseases suitable for monobody therapeutics. In 2024, China registered a 25% rise in monobody-related patent filings and a growing number of public-private partnerships focusing on next-generation biologics. The region’s substantial manufacturing capabilities and a large patient population offer a fertile ground for market growth, attracting major global players establishing local R&D and production facilities.
Analyst Opinion
The progressive adoption of monobody technology across various therapeutic areas is a pivotal demand-side indicator driving the market share. For instance, in 2024, nearly 60% of investigational new drug applications incorporating monobody scaffolds targeted oncology, reflecting a strong inclination towards precision medicine solutions.
Manufacturing innovations allowing scalable monobody production have enhanced supply-side dynamics. Bioprocessing facilities reported a 25% increase in yield optimization in 2025 compared to 2023, significantly reducing the cost and time-to-market for monobody biologics.
Increasing clinical trials focused on monobody conjugates across autoimmune diseases demonstrate growing market scope. Recent data revealed that efforts toward monobody-based immunomodulatory drugs surged by 35% in 2024, indicating expanding market revenue streams beyond cancer treatment.
Regional import-export flows of monobody components indicate expanding global trade dynamics. North America exhibited a 20% rise in monobody reagent imports in 2024, chiefly supplying biopharmaceutical companies scaling R&D activities, underscoring strong market growth potential.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 1.45 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 16.5% | 2033 Value Projection: | USD 4.12 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Novo Nordisk A/S, Egalet Corporation, Boehringer Ingelheim GmbH, Genentech (Roche), UCB Pharma, Biogen Inc., Eli Lilly and Company, Regeneron Pharmaceuticals, Inc., Moderna, Inc., Janssen Pharmaceuticals | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Monobody Based Therapeutic Drugs Market Growth Factors
The rising prevalence of cancer and chronic autoimmune disorders is a key driver for monobody therapeutic adoption as monobody drugs offer enhanced target specificity and reduced immunogenicity. Significant investments by biopharmaceutical firms in 2024 supported clinical pipeline expansions, contributing to substantial market revenue growth. Regulatory agencies streamlining expedited approval pathways for innovative biologics have enabled faster commercialization, thereby increasing market size. Additionally, advancements in protein engineering and synthetic biology have expanded the market scope through the development of novel monobody formats with enhanced stability and multifunctionality.
Monobody Based Therapeutic Drugs Market Development
In December 2024, researchers announced the development of Mirror-Image D-Monobodies targeting oncogenic domains such as BCR::ABL1 SH2 and MCP-1. These “mirror-image” monobodies are designed to resist natural proteases, potentially extending their circulation half-life and improving stability compared with conventional monobodies. This advancement represents a significant step toward more durable biologic therapeutics for cancer and inflammatory diseases.
In October 2024, a study demonstrated the use of Modular Nanotransporters (MNTs) to deliver an anti-Keap1 monobody directly into the cytosol of cells. By blocking Keap1-mediated oxidative stress pathways, this approach showed potential in protecting against liver intoxication, highlighting the promise of intracellular monobody delivery for targeted therapeutic interventions.
Key Players
Leading Companies of the Market
Egalet Corporation
Boehringer Ingelheim GmbH
Genentech (Roche)
UCB Pharma
Biogen Inc.
Eli Lilly and Company
Regeneron Pharmaceuticals, Inc.
Moderna, Inc.
Janssen Pharmaceuticals
Several leading companies have adopted aggressive innovation-focused growth strategies such as expanding R&D collaborations and licensing agreements. For example, a strategic alliance in 2024 between a biotechnology pioneer and a pharmaceutical giant led to a 30% acceleration in regulatory approvals of monobody therapeutics. Another player implemented AI-driven design platforms in 2025, resulting in a 20% reduction in candidate screening timelines, enhancing market position significantly.
Monobody Based Therapeutic Drugs Market Future Outlook
The future outlook for the Monobody Based Therapeutic Drugs Market is shaped by the increasing demand for next-generation biologics that can address intracellular targets and complex protein–protein interactions that remain inaccessible to traditional antibodies. Continued advances in computational protein design, synthetic biology, and targeted delivery technologies are expected to accelerate clinical development and improve therapeutic efficacy. Pharmaceutical companies are likely to expand partnerships with biotechnology firms specializing in scaffold proteins to diversify biologics pipelines.
Regulatory familiarity with non-antibody protein therapeutics is also improving, which is expected to reduce development uncertainty. As precision oncology, rare disease treatment, and immune modulation gain importance, monobodies are expected to play a growing role as adaptable, high-specificity therapeutic agents.
Monobody Based Therapeutic Drugs Market Historical Analysis
The Monobody Based Therapeutic Drugs Market emerged from advances in protein engineering and scaffold-based drug design that gained momentum in the early 2000s, when limitations of conventional monoclonal antibodies—such as large molecular size, complex manufacturing, and restricted tissue penetration—became increasingly apparent. Early academic research demonstrated that monobodies, derived from fibronectin type III domains, could be engineered to bind targets with antibody-like specificity while offering superior stability and modularity.
Initial development was largely confined to research tools and intracellular target validation, as regulatory pathways for non-antibody protein therapeutics were still evolving. Over time, improvements in recombinant expression systems, rational protein design, and high-throughput screening enabled pharmaceutical developers to advance monobody candidates into preclinical pipelines. The growing focus on precision medicine, particularly in oncology and immunology, further supported the transition of monobodies from experimental molecules to clinically relevant therapeutic candidates.
Sources
Primary Research Interviews:
Biotech researchers
Protein engineers
Pharmaceutical scientists
Databases:
NIH Drug Databases
FDA Biologics Data
ClinicalTrials.gov
Magazines:
Nature Biotechnology
Genetic Engineering & Biotechnology News
BoPharma Dive
Drug Discovery Today
Pharmaceutical Technology
Journals:
Nature Medicine
Journal of Biological Chemistry
Molecular Therapy
Clinical Cancer Research
Trends in Pharmacological Sciences
Newspapers:
Financial Times (Biotech)
Reuters Pharma
Bloomberg Life Sciences
The Guardian (Science)
The Wall Street Journal (Health)
Associations:
Biotechnology Innovation Organization
International Society for Biologics
American Society of Clinical Oncology
EMAFDA
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients