Global molecular quality control market is estimated to be valued at USD 223.3 Mn in 2025 and is expected to reach USD 365.9 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 7.3% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The molecular quality control industry can witness positive growth during the forecast period due to rising incidence of infectious diseases and increasing application of molecular diagnostic techniques for diagnosis. Moreover, increasing focus on improving quality of diagnostic tests and growth in biotechnology and pharmaceutical industries can also aid the market growth. However, high cost of these quality control kits may hamper the market growth to during the forecast period. New product launches with advanced technologies and growing adoption in developing countries are expected to offer lucrative opportunities for market players.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
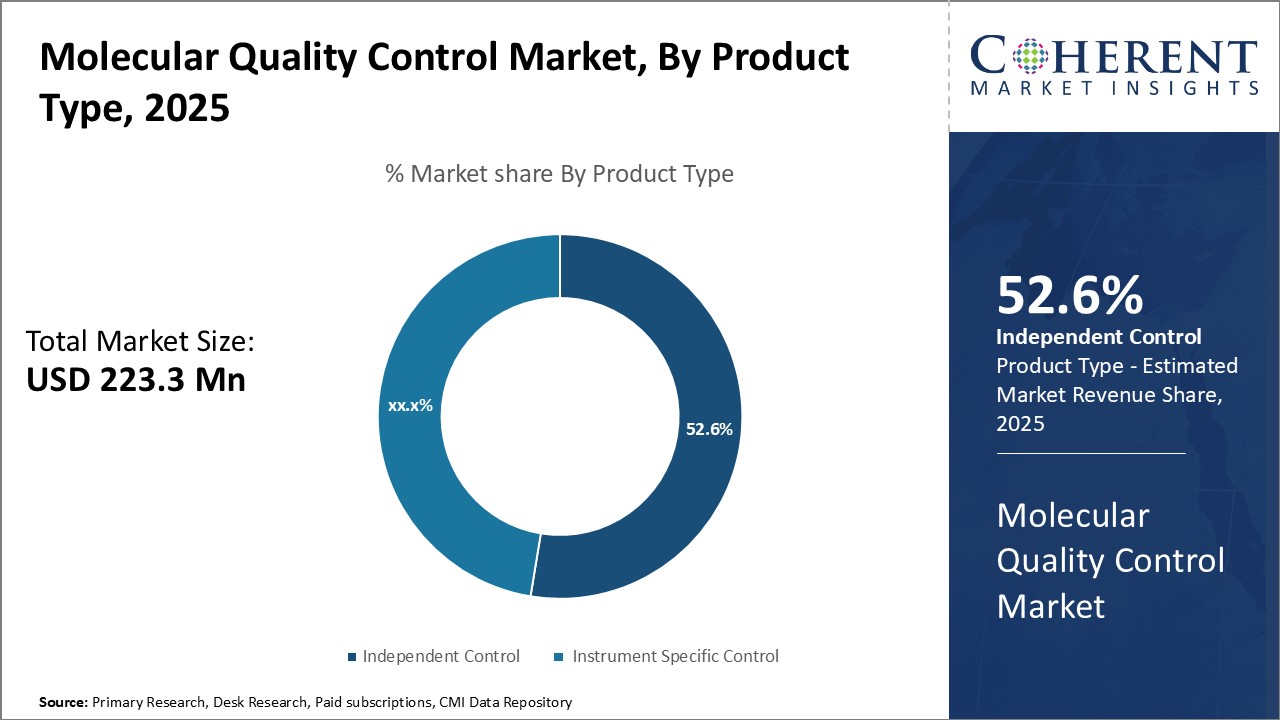
Insights By Product Type - Independent control segment dominates due to crucial quality control needs
In terms of product type, independent control segment is estimated to contribute the highest market share of 52.6% in 2025, owing to its necessity across various molecular testing applications. Independent controls are not specific to any instrument or assay and can be used universally to validate assay performance. These play a pivotal role in ensuring assay accuracy and reproducibility. Compared to instrument-specific controls, independent controls have wider applicability and allow laboratories to check multiple platforms with a single control sample.
Insights By Analyte Type - Ensuring single analyte accuracy boosts demand for single-analyte controls
In terms of analyte type, single-analyte controls segment is estimated to contribute the highest market share of 53.6% in 2025, owing to the need for precision in molecular testing of individual analytes. Single-Analyte Controls contain only one purified analyte, enabling targeted and accurate quality assessment of a specific molecular marker or gene sequence. These are optimised to check the analytical measurement of individual targets without interference from other components.
Insights, By Application - Infectious disease diagnostics leverages controls for improved sensitivity
In terms of application, infectious disease diagnostics segment is estimated to contribute the highest market share of 34.62% in 2025, owing to the crucial role of controls in enhancing test sensitivity. Early and accurate diagnosis of infectious diseases optimizes clinical outcomes. Molecular quality controls catering to infectious disease testing applications ensure correct identification of pathogens down to the lowest detection limits. These certified tests are performing as intended before patient sample analysis. This helps reduce the chances of false negatives.
Need a Different Region or Segment? Download Free Sample
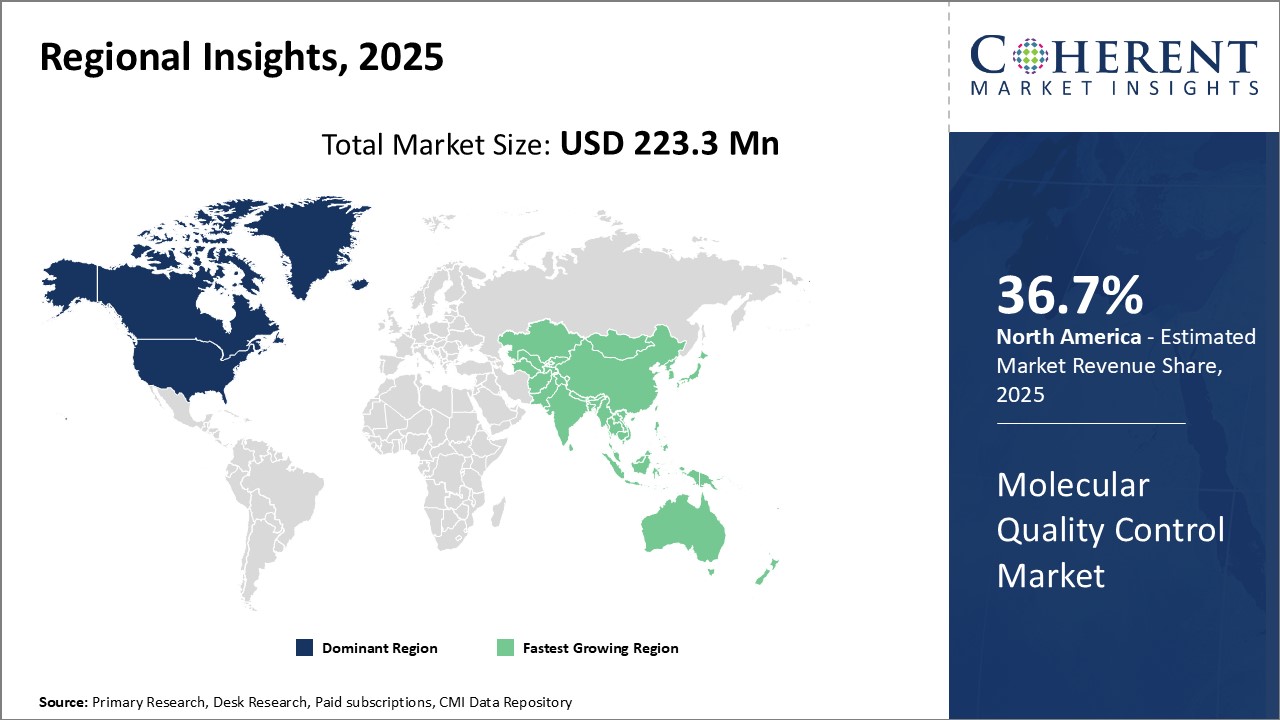
Dominating Region- North America
North America dominates the molecular quality control industry with an estimated market share of 36.7% in 2025, due to robust research funding and presence of leading global players like Bio-Rad Laboratories, Inc. in the region. High adoption of precision medicine and companion diagnostics along with advanced healthcare infrastructure boosts the market growth.
Fastest-Growing Region- Asia Pacific
Asia Pacific region exhibits the fastest growth due to increasing healthcare expenditure, growing focus on pharmaceutical and biotech R&D, and expanding patient pool in countries like China and India. Improving regulatory guidelines are also encouraging global players to tap into opportunities in Asia Pacific.
Molecular Quality Control Market Outlook for Key Regions
U.S.- Dominance Driven by Large Patient Pool and High Infectious Disease Prevalence
The U.S. dominates the North America’s molecular quality control industry due to various factors. The country has a large and growing patient pool, particularly in infectious diseases such as HIV. In August 2025, according to the U.S. Department of Health & Human Services, approximately 38,000 new HIV infections occur annually in the U.S. and its dependent areas, leading to a rapid spread of various infectious diseases, including meningitis and urinary tract infections.
Japan- Growth Driven by Advancements in Diagnostic Technologies and Regulatory Approvals
Japan’s molecular quality control market is growing due to advancements in diagnostic technologies and stringent regulatory standards. For instance, on June 13, 2024, ELITechGroup announced the successful approval of its Blood Borne Virus (BBV) panel by Japan’s Pharmaceuticals and Medical Devices Agency (PMDA). The BBV panel includes assays for Human Immunodeficiency Virus (HIV-1), Hepatitis B Virus (HBV), and Hepatitis C Virus (HCV), offering fully automated Polymerase Chain Reaction (PCR) solutions for monitoring these viruses.
India- Market Leadership Fueled by Growing Healthcare Infrastructure and Rising Diagnostic Demand
India is dominant in the molecular quality control market due to its growing healthcare infrastructure, rising demand for diagnostic solutions, and increasing investments in biotechnology and pharmaceuticals. In July 2020, Thermo Fisher Scientific introduced the Thermo Scientific MAS Omni Infectious Disease quality control sets for serological assays, covering HIV 1&2, Hepatitis B & C, Syphilis, and HTLV I/II. These third-party, independent external controls help assess assay performance and support the growing demand for serology testing in infectious disease diagnosis, monitoring, and treatment.
Get actionable strategies to beat competition: Download Free Sample
Top Strategies Followed by Global Molecular Quality Control Industry Players
Emerging Startups in the Global Molecular Quality Control Market
Innovative Technologies- The industry has witnessed entry of startups developing smart solutions based on emerging technologies like Cloudink and Precytron. Cloudink creates miniature wireless biosensors integrated with machine learning algorithms. These sensors can monitor the performance of molecular diagnostic kits and instruments in real-time. Precytron has pioneered a handheld molecular diagnostics platform equipped with an AI-powered sensor. Adoption of such advanced technologies could transform quality monitoring processes and drive efficiencies.
Sustainable Solutions- Sustainability is a major focus among emerging startups. Firms like PBC and Moleculars21 are introducing eco-friendly quality control products made from biodegradable or recycled materials. PBC produces biosynthetic controls from plant-based polymers that degrade harmlessly. Moleculars21 recovers PCR plastics from used diagnostic kits and converts them into new QC samples. Such initiatives are in line with the industry's environmental objectives and support the growth of startups.
Key Takeaways from Analyst
Molecular Quality Control Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 223.3 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.3% | 2032 Value Projection: | USD 365.9 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
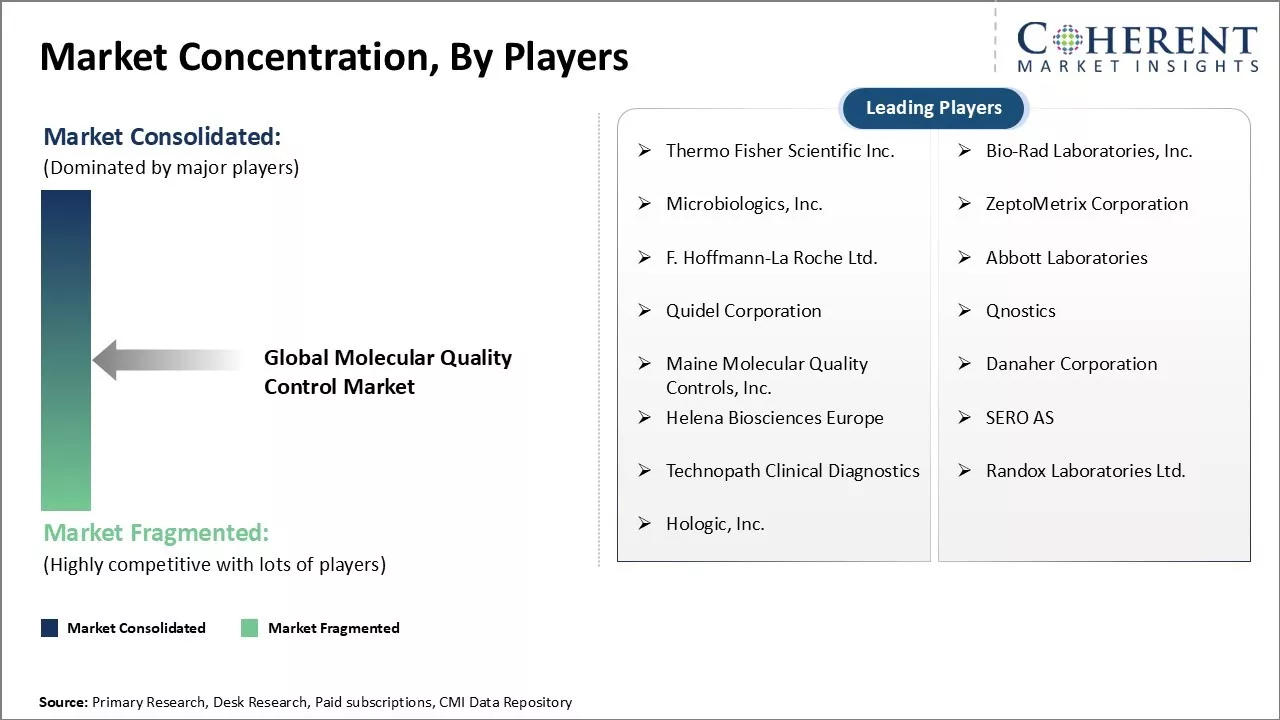
Thermo Fisher Scientific Inc., Bio-Rad Laboratories, Inc., Microbiologics, Inc., ZeptoMetrix Corporation, F. Hoffmann-La Roche Ltd., Abbott Laboratories, Quidel Corporation, Qnostics, Maine Molecular Quality Controls, Inc., Danaher Corporation, Helena Biosciences Europe, SERO AS, Technopath Clinical Diagnostics, Randox Laboratories Ltd., and Hologic, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
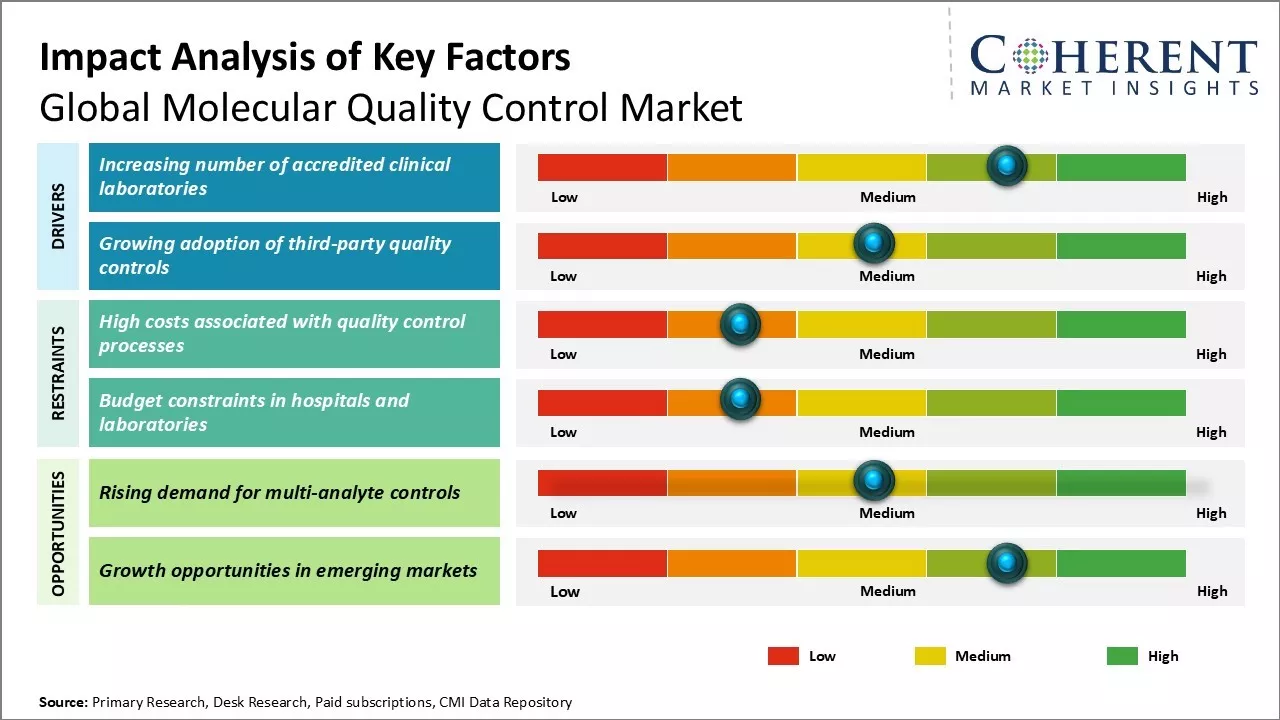
Market Driver- Increasing number of accredited clinical laboratories
Global molecular quality control market growth is driven by growing number of accredited clinical laboratories worldwide, which are increasingly adopting advanced molecular diagnostic technologies. These advancements enable laboratories to offer highly sensitive and specific tests, enhancing diagnostic accuracy. For instance, in May 2024, Bruker Corporation acquired ELITechGroup for USD 870 million, integrating ELITech’s automated sample-to-answer platforms (InGenius and BeGenius), which specializes in esoteric infectious disease testing for mid-sized hospitals. This integration highlights the rising demand for innovative molecular diagnostics systems in accredited laboratories.
Market Challenge- High costs associated with quality control processes
Major challenges faced by global molecular quality control market includes high costs associated with quality control processes. Implementing quality control protocols requires continuous monitoring and testing, which involves significant costs in terms of equipment, reagents, and manpower. Molecular diagnostic assays need to be standardized and validated on a regular basis through rigorous quality testing using multiple controls and calibrators. This adds to research and development expenses for market players.
Market Opportunity- Rising demand for multi-analyte controls
Major opportunity in the global molecular quality control market includes rising demand for multi-analyte controls. Contemporary molecular diagnostics involves multiplexing of assays to simultaneously detect and quantify several biomarkers or analytes. This allows for streamlined and high-throughput testing. Consequently, there has been increasing demand for controls capable of verifying the performance of complex multiplex assays. Multi-analyte controls help validate multiple targets in a single reaction and analyse interferences.
What growth in global molecular quality control market mean for different stakeholders?
Molecular quality control market has multiple players with varied designations and offers multiple opportunities based on their scope of operations.
|
Key Clinical Diagnostics Stakeholder |
Opportunities Due to Molecular Quality Control Industry Growth |
|
Diagnostic Equipment Manufacturers |
Expansion of markets for advanced diagnostic instruments, including PCR machines, immunoassay analysers, and next-generation sequencing systems. |
|
Clinical Laboratories |
Growth in demand for comprehensive diagnostic services, including molecular testing, genetic screening, and personalized health assessments. |
|
Healthcare Providers |
Enhanced ability to offer personalized medicine and early disease detection, improving patient outcomes and expanding clinical services. |
|
Healthcare IT Firms |
Expanding into diagnostic data management, laboratory information systems (LIS), and integration of diagnostic data with electronic health records. |
|
Medical Device Companies |
Collaborating with diagnostic firms to develop integrated diagnostic devices and expanding into new areas such as wearable diagnostics. |
|
Pharmaceutical Companies |
Leveraging diagnostic tools for drug development and personalized medicine, enhancing drug efficacy and patient stratification in clinical trials. |
|
Biotech Firms |
Developing and commercializing biomarkers and companion diagnostics, supporting personalized treatment plans and targeted therapies. |
|
Venture Capitalists in Diagnostics |
Investment opportunities in startups developing innovative diagnostic technologies, including AI-driven diagnostics and home testing solutions. |
|
Private Equity Investors |
Potential to invest in diagnostic companies with cutting-edge technologies and scalable business models, driving industry consolidation. |
|
Diagnostic Consultants |
Providing expertise in regulatory compliance, market access, and the commercialization of new diagnostic products and services. |
|
Retail Pharmacies |
Offering diagnostic services and home testing kits, expanding customer care services and increasing market reach. |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients