Mitochondrial Disorders Treatment Market Size and Forecast – 2025 – 2032
The Global Mitochondrial Disorders Treatment Market size is estimated to be valued at USD 1.2 billion in 2025 and is expected to reach USD 2.6 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 11.3% from 2025 to 2032.
Global Mitochondrial Disorders Treatment Market Overview
Mitochondrial disorder treatments target cellular energy deficiencies caused by dysfunctional mitochondria. Current therapeutic products include coenzyme Q10, L-carnitine, and antioxidant supplements that enhance mitochondrial function and reduce oxidative stress. Experimental drugs focus on modulating mitochondrial biogenesis and stabilizing energy metabolism. New formulations also explore gene therapy and enzyme replacement approaches to address the underlying genetic mutations responsible for these disorders.
Key Takeaways
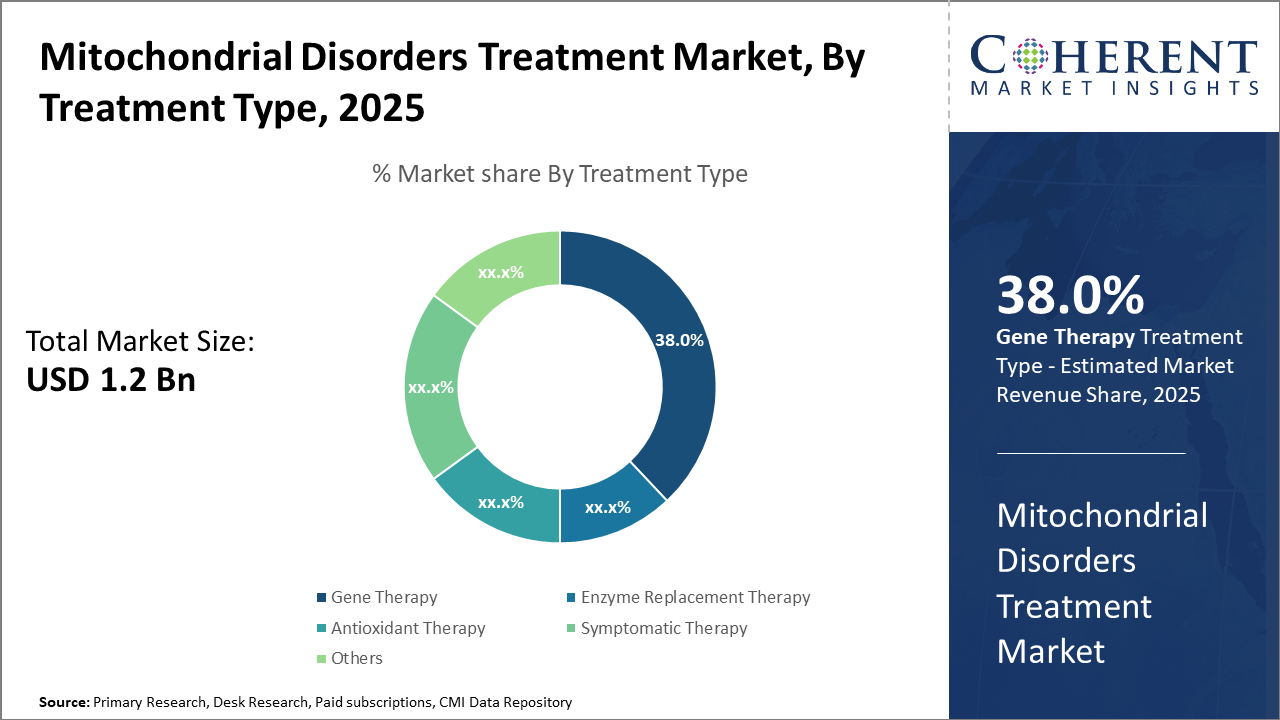
The Gene Therapy segment dominates the treatment landscape, accounting for 38% of the market share, driven by clinical successes and increasing regulatory approvals.
Primary Mitochondrial Myopathies represent the largest disease segment, supported by rising diagnostic accuracy and personalized treatment options.
Hospitals and Clinics remain the principal end-users, comprising over 50% of treatment delivery owing to established infrastructure and integrated patient care models.
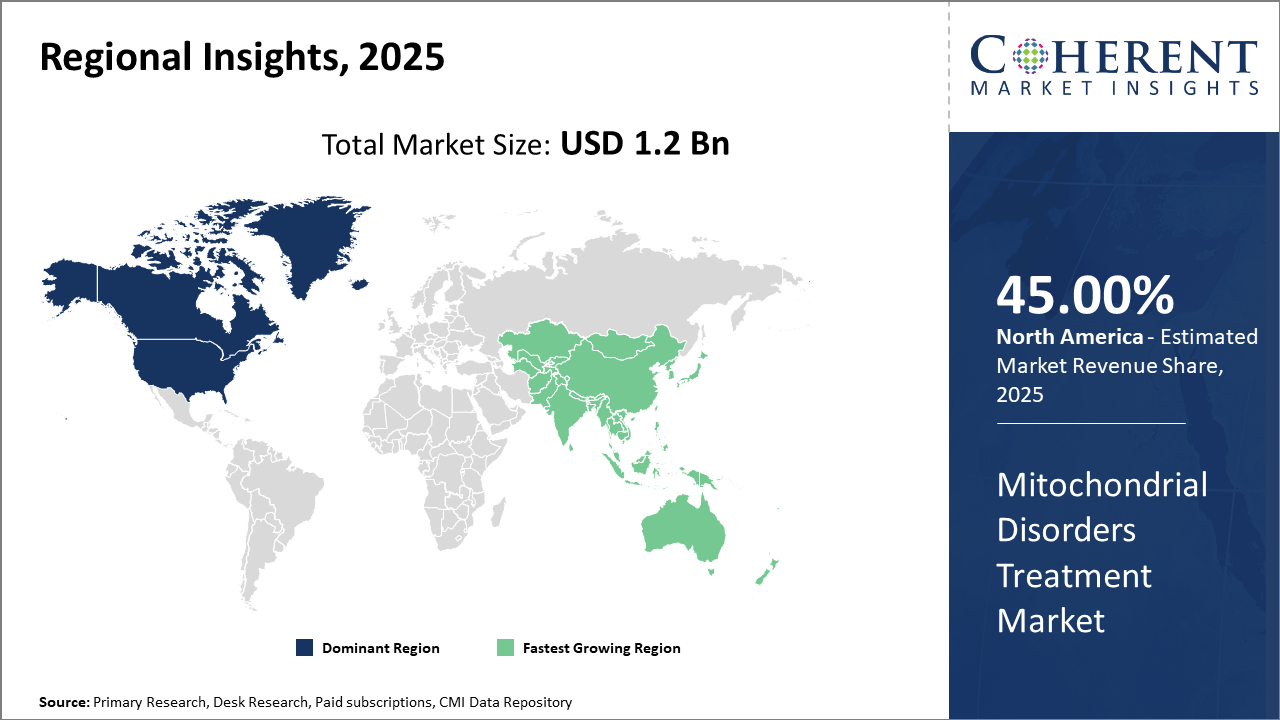
Regionally, North America leads with a commanding market share of approximately 45%, attributed to advanced healthcare infrastructure and significant R&D investments.
Europe follows closely, with regulatory harmonization enhancing market dynamics. Asia Pacific emerges as the fastest-growing region with a CAGR notably higher than 13%, driven by expanding healthcare expenditure, increasing patient awareness, and government-led rare disease initiatives.
Mitochondrial Disorders Treatment Market Segmentation Analysis
To learn more about this report, Download Free Sample
Mitochondrial Disorders Treatment Market Insights, By Treatment Type
Gene Therapy dominates the market share with 38%. This dominance is driven by successful clinical outcomes, regulatory approvals, and increasing investments in gene editing platforms, making it the most attractive segment for innovation and business growth. The fastest-growing subsegment is Enzyme Replacement Therapy, propelled by ongoing clinical trials aimed at reducing side effects and improving patient quality of life. Antioxidant Therapy, serving as an adjunct, remains steady due to its symptomatic relief benefits. Symptomatic Therapy caters to managing complications but lacks long-term disease modification potential.
Mitochondrial Disorders Treatment Market Insights, By Disease Type
Primary Mitochondrial Myopathies hold the largest share due to higher diagnosis rates and developed treatment protocols. Its dominance is supported by extensive genomic screening and diagnostic advancements, facilitating early intervention. The fastest-growing subsegment is Leigh Syndrome, gaining attention because of novel gene therapy candidates showing promising efficacy in clinical trials. MELAS and MERRF Syndromes maintain modest growth, primarily driven by symptomatic treatment demands and increasing case identification.
Mitochondrial Disorders Treatment Market Insights, By End-User
Hospitals and Clinics dominate the market share due to their established infrastructure, multidisciplinary teams, and capability to administer complex mitochondrial disorder treatments, especially in urban areas. The fastest-growing subsegment is Specialty Clinics, which are expanding rapidly as centers of excellence focusing on precision therapies and personalized patient management. Research Institutes play a vital role by facilitating trials and discovery, though with smaller direct revenue contributions.
Mitochondrial Disorders Treatment Market Trends
Market trends in mitochondrial disorders treatment are emphasizing precision medicine enhanced by genomic and proteomic advances.
For instance, 2025’s approvals of novel gene therapies have showcased the potential for disease modification instead of symptomatic treatment alone.
Digital integration, including AI-aided diagnostics and remote patient monitoring, further contributes to streamlined care pathways and improved clinical outcomes.
Another noteworthy trend is the increasing patient advocacy involvement, which is influencing market dynamics by accelerating clinical trial recruitment and policy reforms.
Mitochondrial Disorders Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Mitochondrial Disorders Treatment Market Analysis and Trends
In North America, the dominance in the Mitochondrial Disorders Treatment market is attributable to robust healthcare infrastructure, substantial government funding, and a high concentration of market players. The region holds approximately 45% of the market share, fueled by favorable reimbursement environments and advanced genetic testing availability. Leading U.S. companies like Ultragenyx and Sarepta Therapeutics significantly contribute through innovative therapies and strategic alliances.
Asia Pacific Mitochondrial Disorders Treatment Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with a CAGR exceeding 13%, supported by increasing healthcare investments and rising disease awareness. Countries such as China and India are spearheading growth due to expanding genetic screening programs and favorable government policies promoting rare disease frameworks. Additionally, growing presence of multinational pharmaceutical companies and local biotech startups accelerates the business growth and market development in this region.
Mitochondrial Disorders Treatment Market Outlook for Key Countries
USA Mitochondrial Disorders Treatment Market Analysis and Trends
The USA’s mitochondrial disorders treatment market remains the global frontrunner, propelled by the concentration of advanced clinical trials and pioneering gene therapy companies. In 2024, the FDA approvals for two novel gene-editing treatments dramatically enhanced treatment options, boosting market revenue. Furthermore, extensive federal and private funding initiatives for rare diseases have fortified the country’s research capabilities. This creates a fertile ground for continuous innovation, helping the U.S. maintain its industry share dominance and influence global market trends.
Japan Mitochondrial Disorders Treatment Market Analysis and Trends
Japan's market is gaining significant traction owing to increasing government support for rare disease treatments and integration of cutting-edge molecular diagnostics. Healthcare policies encouraging orphan drug development, along with strong collaborations between academia and biotech firms, have accelerated product pipelines. In 2025, emerging therapies witnessed rapid uptake in Japanese hospitals, contributing to positive market growth. These developments position Japan as a critical player driving the Asia Pacific expansion.
Analyst Opinion
Production Capacity Expansion: The global demand for mitochondrial disorders treatment is correlated with the enhanced capacity of pharmaceutical manufacturing units specializing in orphan drugs. In 2024, production ramp-ups in North America accounted for a 15% increase in drug availability compared to 2023, aligning with market growth trajectories. Increased capacity optimization in APAC, particularly India and China, is also lowering treatment costs and improving accessibility.
Pricing Trends and Reimbursement Policies: Pricing remains a significant market driver due to the high cost of advanced therapies such as gene editing and personalized mitochondrial treatments. For instance, groundbreaking approvals in 2024 introduced therapies priced upwards of USD 500,000 per patient, exerting pressure for broader insurance coverage. Recent modifications in healthcare reimbursement policies in Europe and the U.S. have enabled over 20% faster patient access, positively influencing market revenue.
Demand Across Therapeutic Areas: Beyond primary mitochondrial disorders, promising applications are emerging in neurology and metabolic diseases that present mitochondrial dysfunction symptoms. This expanded use-case led to an increase of approximately 18% in treatment adoption rates in 2025 among neurologists in developed economies, as reported by hospital registries. Furthermore, pediatric treatment demand is surging due to early diagnostic advancements.
Microlevel Indicators - Diagnostic Innovation: The introduction of next-generation sequencing (NGS) platforms improved clinical diagnostics, resulting in a 25% reduction in diagnostic turnaround times between 2023 and 2025. This accelerates patient stratification for targeted therapies, directly contributing to increased market size. Research centers adopting AI-driven genomic analytics have reported a 30% boost in identifying mitochondrial mutation patterns, thereby catalyzing market expansion.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 1.2 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 11.3% | 2032 Value Projection: | USD 2.6 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Ultragenyx Pharmaceutical Inc., Sanofi Genzyme, BioMarin Pharmaceutical Inc., Sarepta Therapeutics, Stealth BioTherapeutics, PTC Therapeutics, Horizon Therapeutics, Mitsui Chemicals, Pfizer Inc., Roche Holding AG, Novartis AG. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Mitochondrial Disorders Treatment Market Growth Factors
Increasing genetic testing adoption facilitates early diagnosis, driving demand for effective mitochondrial disorders treatment options globally. For instance, genomic screening initiatives in Europe led to a 20% growth in diagnosed cases in 2024, subsequently boosting treatment uptake. Breakthroughs in gene therapy modalities, such as CRISPR and viral vector delivery, have resulted in several promising candidates entering advanced clinical trials, pushing the innovation curve and market growth faster than anticipated.
Rising government support with increased funding in rare disease treatment programs, especially in the U.S. and Japan, has accelerated market penetration. The U.S. Orphan Drug Act incentives led to a 15% surge in new drug development for mitochondrial treatments in 2025. Enhanced awareness campaigns and patient advocacy efforts have expanded market scope by improving patient referrals and adherence, with notable increases in patient registries across North America and Europe, recorded at a 17% rise in 2024.
Mitochondrial Disorders Treatment Market Development
In September 2025, Stealth BioTherapeutics received accelerated approval from the U.S. FDA for FORZINITY (elamipretide), marking the first-ever approved therapy for Barth syndrome and the first mitochondria-targeted treatment to reach the market. FORZINITY is designed to restore mitochondrial structure and function by binding to cardiolipin, a key phospholipid essential for energy production within cells. This approval represents a major milestone in mitochondrial medicine, offering a new therapeutic option for patients with Barth syndrome — a rare, life-threatening genetic disorder characterized by cardiac and skeletal muscle weakness.
In February 2025, Reneo Pharmaceuticals announced that its investigational therapy REN001 received orphan drug designation from the U.S. FDA for the treatment of primary mitochondrial myopathies (PMM). REN001 is a selective PPARδ agonist that targets impaired energy metabolism in skeletal muscle, with the goal of improving exercise tolerance and reducing fatigue in patients with mitochondrial dysfunction. The designation provides Reneo with regulatory incentives, including market exclusivity and tax credits for clinical development.
Key Players
Leading Companies of the Market
Ultragenyx Pharmaceutical Inc.
Sanofi Genzyme
Biomarin Pharmaceutical Inc.
Sarepta Therapeutics
Stealth BioTherapeutics
PTC Therapeutics
Horizon Therapeutics
Mitsui Chemicals
Pfizer Inc.
Roche Holding AG
Novartis AG
Several leading companies are aggressively adopting expansion strategies, such as the 2024 collaboration between Ultragenyx Pharmaceutical Inc. and Sanofi Genzyme to develop next-gen gene therapies, resulting in a 12% increase in their combined market share within a year. Additionally, Biomarin Pharmaceutical Inc. implemented a licensing agreement with a South Korean biotech firm in 2025, enabling penetration into APAC markets with a recorded 9% growth in regional revenue. Continuous enhancement of manufacturing capabilities and clinical trial collaborations remain key competitive strategies within these organizations.
Mitochondrial Disorders Treatment Market Future Outlook
The market’s trajectory points toward precision therapeutics and molecular innovation. As next-generation sequencing becomes routine, earlier and more accurate diagnosis will boost patient inclusion in clinical studies. Emerging therapies, including gene replacement, mitochondrial transfer, and nucleic acid modulation, are expected to redefine treatment paradigms. Investment from rare disease-focused biotech firms and regulatory incentives for orphan drugs will accelerate approvals. Global collaboration among researchers and advocacy groups will continue to expand awareness and funding, positioning the market for steady long-term growth.
Mitochondrial Disorders Treatment Market Historical Analysis
The mitochondrial disorders treatment market has long been constrained by diagnostic challenges, limited therapeutic options, and a fragmented patient base. Historically, treatment strategies relied on supportive care—vitamins, antioxidants, and symptom-based interventions—due to the complexity of mitochondrial pathophysiology. However, progress in genomics and molecular diagnostics during the last two decades has significantly improved disease identification and classification. Academic research collaborations and public-private partnerships laid the foundation for targeted drug development, marking a shift from symptomatic to disease-modifying approaches. The gradual increase in clinical trials and orphan drug designations has brought more visibility and investment into this once-overlooked therapeutic space.
Sources
Primary Research interviews:
Neurologists
Genetic Researchers
Clinical Pharmacologists
Biotech R&D Experts
Databases:
NIH Rare Diseases Database
Orphanet
PubMed
GlobalData Life Sciences Database
Magazines:
PharmaVoice
Biopharma Reporter
Rare Disease Advisor
Nature Medicine
Journals:
Mitochondrion
Journal of Inherited Metabolic Disease
Molecular Therapy
Journal of Rare Diseases Research & Treatment
Newspapers:
The Hindu (Health)
The Economic Times (Pharma)
Financial Times (Science)
The Guardian (Healthcare)
Associations:
United Mitochondrial Disease Foundation
European Mitochondrial Disease Network
National Organization for Rare Disorders
American Society of Human Genetics
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients