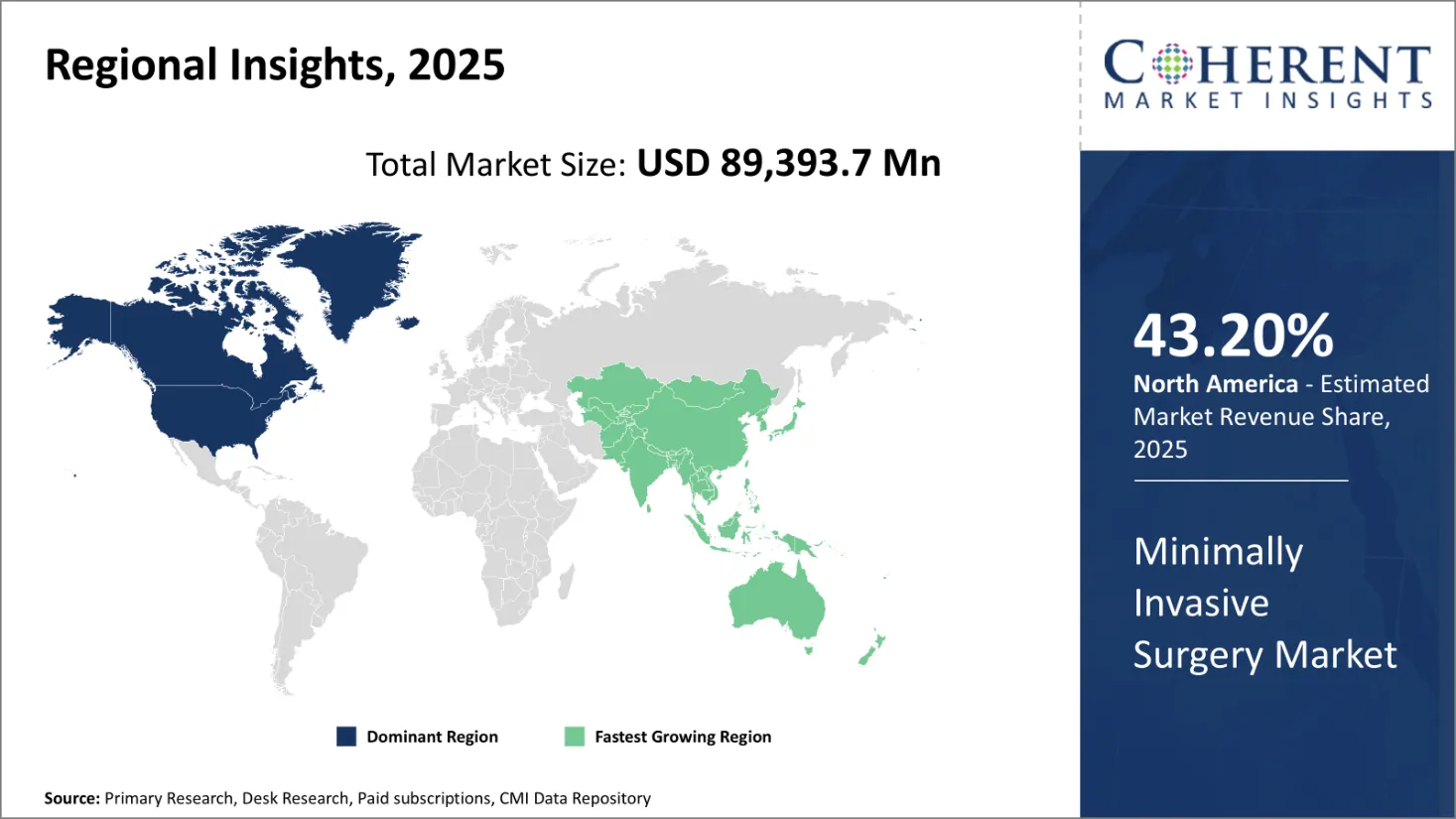
Minimally Invasive Surgery Market size is estimated to be valued at USD 89,393.7 Mn in 2025 and is expected to reach USD 1,85,595.6 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 11% from 2025 to 2032.
The Minimally Invasive Surgery (MIS) market value is experiencing robust growth, driven by the increasing demand for procedures that offer reduced trauma, quicker recovery, and lower complication risks compared to traditional open surgeries. Usage of minimally invasive surgery for the treatment of major chronic diseases is expected to drive the global minimally invasive surgery market growth over the forecast period.
For instance, the adoption of minimally invasive transcatheter aortic valve replacement (TAVR) has significantly increased for patients with severe aortic stenosis. According to the American College of Cardiology, as of 2025, over 80% of eligible elderly patients in the U.S. are now treated with TAVR rather than open-heart surgery, owing to its advantages in minimizing postoperative complications and expediting recovery—highlighting a clear trend toward MIS in chronic disease management.
|
Event |
Description and Impact |
|
EU Medical Device Regulations (MDR) Implementation |
|
|
FDA AI Integration for Regulatory Reviews |
|
|
Global Supply Chain Disruptions |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The surgical devices segment is expected to dominate with 55.0% share of the global minimally invasive surgery market over the forecast period. This segment includes ablation devices, electrosurgical devices, and medical robotic systems. The segment’s leading position is primarily driven by the growing use of endoscopy devices, which are integral to various minimally invasive procedures due to their ability to provide high-resolution imaging and precise navigation in confined anatomical areas. The expanding adoption of robotic-assisted surgical systems is further reinforcing the demand for advanced surgical tools, particularly in complex surgeries that require enhanced dexterity and precision.
Meanwhile, the monitoring & visualization devices and endoscopy devices segments are also poised for significant growth. These devices play a crucial role in enhancing procedural accuracy and minimizing intraoperative risks. Innovations in imaging technologies, such as 3D visualization and high-definition optics, are reshaping intraoperative guidance, making them indispensable tools for modern minimally invasive interventions.
Among surgical types, the cardiac surgery segment is expected to lead the global minimally invasive surgery market throughout the forecast period. This dominance is attributed to the rising global prevalence of cardiac disorders, which is fueling the demand for less invasive alternatives to conventional open-heart surgeries. Minimally invasive cardiac procedures offer the benefits of shorter recovery times, fewer complications, and reduced hospital stays, making them increasingly preferred by both patients and healthcare providers.
The orthopedic surgery segment is also projected to witness substantial growth over the coming years. The growing burden of musculoskeletal conditions, particularly osteoarthritis among the elderly population, is a key driver. For example, according to the World Health Organization, a significant portion of the global population aged 60 years and older suffers from osteoarthritis, contributing to a higher volume of joint replacement and repair procedures using minimally invasive techniques.
With the increasing application of MIS across other specialties such as gynecological, gastrointestinal, and cosmetic surgery, the surgery segment as a whole presents the highest potential to propel market growth, as healthcare systems increasingly prioritize procedures that reduce trauma and enhance patient outcomes.
To learn more about this report, Download Free Sample
North America is projected to dominate with 43.20% share of the global minimally invasive surgery market over the forecast period, supported by the region’s advanced healthcare infrastructure, strong adoption of innovative surgical technologies, and rising prevalence of chronic diseases—particularly cardiovascular conditions. The U.S., in particular, continues to lead in the use of robotic-assisted and image-guided minimally invasive procedures, driven by patient demand for faster recovery and lower procedural risks.
According to the New York State Department of Health (August 2025), approximately 697,000 people in the U.S. die from cardiac disease annually, underscoring a significant need for less invasive cardiac interventions. These high disease burdens, coupled with increasing investments in surgical innovation and training, are reinforcing North America’s leading position in the market. However, market growth in the region may be moderated by high procedural and device costs, as well as regulatory complexities.
Increasing prevalence of orthopedic diseases is expected to drive the global minimally invasive surgery market growth over the forecast period. For instance, in July 2022, according to World Health Organization (WHO), approximately 1.71 billion people have musculoskeletal conditions worldwide.
Increasing number of cardiac surgeries is expected to drive the global minimally invasive surgery market growth over the forecast period. For instance, in June 2022, according to an article published by National Institute of Health, more than 2 million people underwent open-heart surgery to treat various heart problems worldwide, every year.
Global minimally invasive surgery market growth can be hindered by disadvantages associated with minimally invasive surgery. For instance, according to Spine Institute of North America, a leading spine and pain specialist, the main disadvantage of minimally invasive surgery is that it requires a higher level of training, and thus, insufficient training can lead to complications such as perforation of the gallbladder, biliary leak, bile duct injury, biliary stricture, etc.
Hence, reducing the disadvantages associated with minimally invasive surgery will assess in the growth of the global minimally invasive surgery market.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 89,393.7 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 11% | 2032 Value Projection: | USD 1,85,595.6 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Abbott, ArthroCare Corporation, Integer Holdings Corporation, Zimmer Biomet, DePuy Synthes, Inc., Conmed Corporation, GE Healthcare, Given Imaging Ltd., Intuitive Surgical, Inc., Medtronic plc., NuVasive, Inc., Philips Healthcare, InMode Ltd., Titan Medical Inc., Fractyl Inc., Biom’up SA, and Integrated Endoscopy |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
In surgery, cardiac surgery held a dominant segment in North America region due to increasing prevalence of cardiac disorders. For instance, in July 2020, according to Healthline Media LLC, a consumer health publisher, about 647,000 people die due to cardiac disease every year that makes it the leading cause of death in the U.S.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients