Middle East And Turkey Hemophilia Treatment Market is estimated to be valued at USD 825.6 Mn in 2025 and is expected to reach USD 1,123.5 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 4.5% from 2025 to 2032.
Analysts’ Views on Middle East and Turkey Hemophilia Treatment Market:
Increasing technological advancement for development of new treatment for hemophilia by key market players is expected to drive the market growth over the forecast period. For instance, on March 2, 2023, Sanofi S.A., a pharmaceutical company, announced the completion of XTEND-Kids Phase 3 study strengthens potential of ALTUVIIIO for treatment of children <12 years of age with hemophilia A. ALUTVIIIO is a first-in-class, high-sustained FVIII therapy that is approved by the US Food and Drug Administration (FDA) for routine prophylaxis, control of bleeding, and perioperative management of bleeding in adults and children.
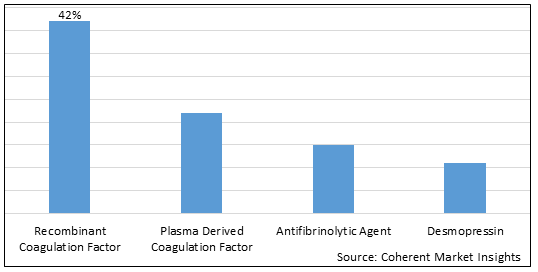
Figure 1. Middle East and Turkey Hemophilia Treatment Market Share (%), By Product Type, 2025
To learn more about this report, Download Free Sample
Middle East and Turkey Hemophilia Treatment Market– Drivers
Increasing Inorganic Growth Strategies Such As Agreement
Increasing inorganic growth strategies such as agreement by key market players to expand their product portfolio is expected to drive the market growth over the forecast period. For instance, in May 2021, CSL Behring, a pharmaceutical company, announced license agreement with UniQure N.V., a healthcare company, for etranacogene dezaparvovec (AMT-061), a novel gene therapy for the treatment of hemophilia B. Etranacogene dezaparvovec is currently in Phase 3 clinical trials for hemophilia B.
Increasing Prevalence Of Hemophilia
Increasing prevalence of hemophilia in Turkey is expected to drive the market growth over the forecast period. For instance, in June 2020, according to data published by the Journal Transfusion and Apheresis Science, in 2018, the percentage of von Willebrand disease (vWd), hemophilia and rare bleeding disorders (RBD) were 40 %, 34 % and 26 %, type-1, type-2 and type-3 vWd were 63 % 17 % and 20 %, hemophilia A and B were 84 % and 16 %, and severe, moderate and mild hemophilia were 48 %, 30 % and 22 %, in Turkish children respectively.
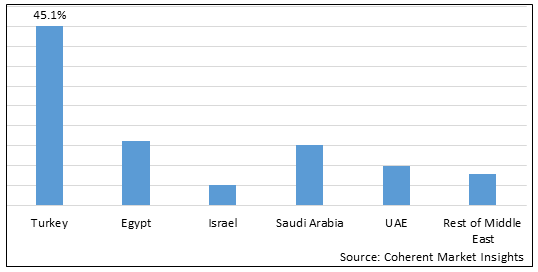
Figure 2. Middle East and Turkey Hemophilia Treatment Market Value (US$ Million), By Country, 2025
To learn more about this report, Download Free Sample
Middle East and Turkey Hemophilia Treatment Market- Country Analysis
Among countries, Turkey is estimated to hold a dominant position in the Middle East and Turkey hemophilia treatment market over the forecast period, due to increasing inorganic growth strategies such as partnership by key market players. For instance, in March 2021, Takeda Pharmaceutical Company Limited., a pharmaceutical company, announced strategic partnership with Enzyre, a developer of diagnostic technology, to accelerate the development of Enzyre’s proprietary platform named Enzypad to enable patients to test their blood coagulation in a home setting that aims to improve the standard of care for patients with bleeding disorders.
Middle East and Turkey Hemophilia Treatment Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others faced problems regarding transportation of drugs from one place to another.
However, the COVID-19 pandemic had a negative impact on the Middle East and Turkey hemophilia treatment market, owing to decrease in patient visits to hospitals during COVID-19. For instance, on March 16, 2023, according to data provided by the National Library of Medicine, there was decrease in the number of visits and hospital admission during the January–October 2020 period after the outbreak of the COVID-19 pandemic. Cancellation of these services negatively affects the patient’s outcome and the future burden of cancer disease. The visits throughout January–October 2020 compared to the pre-pandemic period was decreased by 37.8%, which reached its maximum decline in April –44.3%
Middle East and Turkey Hemophilia Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 825.6 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 4.5% | 2032 Value Projection: | USD 1,123.5 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Takeda Pharmaceutical Company Limited., Sanofi S.A., Octapharma AG, Swedish Orphan Biovitrum AB, Baxter International Inc., Biogen Inc., Bayer AG, CSL Behring, Ferring B.V., Pfizer, Inc., Kedrion, Novo Nordisk A/S, Grifols S.A., Sangamo Therapeutics, Inc., and Spark Therapeutics, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Middle East and Turkey Hemophilia Treatment Market- Segmentation
Middle East and Turkey hemophilia treatment market is segmented into product type, disease indication, and country.
Based on Product Type, the market is segmented into recombinant coagulation factor, plasma derived coagulation factor, antifibrinolytic agents, and desmopressin. Out of which, the recombinant coagulation factor segment is expected to hold a dominant position in the Middle East and Turkey hemophilia treatment market during the forecast period, owing to increasing product approval of recombinant coagulation factor.
Based on Disease Indication, the market is segmented into Hemophilia A, Hemophilia B, and Hemophilia C. Out of which, the Hemophilia A segment is expected to hold a dominant position in the Middle East and Turkey hemophilia treatment market during the forecast period, owing to increasing prevalence of Hemophilia A.
Based on Country, the market is segmented into Turkey, Egypt, Israel, Saudi Arabia, UAE, and Rest of Middle East. Out of which, Turkey is expected to dominate the market over the forecast period, owing to increasing organic growth strategies such as product launches by key players in the country.
Among all segmentation, disease indication segment has the highest potential due to increasing adoption of organic growth strategies such as product approval by regulatory authorities, in order to expand their product portfolio. For instance, in August 2020, BioMarin Pharmaceutical Inc., a pharmaceutical company, announced that the U.S. Food and Drug Administration (FDA) has issued a Complete Response Letter (CRL) to the Company's Biologics License Application (BLA) for valoctocogene roxaparvovec gene therapy for severe hemophilia A on August 18, 2020. The U.S. FDA issues a CRL to indicate that the review cycle for an application is complete, and that the application is not ready for approval in its present form.
Middle East and Turkey Hemophilia Treatment Market- Cross Sectional Analysis
Among disease indication , Hemophilia A segment holds a dominant poistion in Middle East region due to increasing research and development activities by key market players. For instance, in November 2021, Sigilon Therapeutics, a biotechnology company, reported that fibrosed spheres were observed during a retrieval procedure in a patient in its Phase 1/2 study of SIG-001 in severe or moderately severe hemophilia A.
Middle East and Turkey Hemophilia Treatment Market- Key Developments
On February 23, 2023, Sanofi S.A., a healthcare company, announced that the U.S. Food and Drug Administration (FDA) has approved ALTUVIIIO [Antihemophilic Factor (Recombinant), Fc-VWF-XTEN Fusion Protein-ehtl], previously referred to as efanesoctocog alfa, a first-in-class, high-sustained factor VIII replacement therapy and indicated for routine prophylaxis, as well as perioperative management (surgery) for adults and children with hemophilia A.
On January 8, 2023, BioMarin Pharmaceutical Inc., a biotechnology company, announced positive results of its ongoing global Phase 3 GENEr8-1 study of ROCTAVIANTM (valoctocogene roxaparvovec), an investigational one-time gene therapy for the treatment of adults with severe hemophilia A
Middle East and Turkey Hemophilia Treatment Market- Key Trends
Increasing Research and Development Activities By Key Market Players
Increasing research and development activities for hemophilia by key market players, in order to expand their product portfolio is expected to drive the market growth over the forecast period. For instance, in November 2020, UniQure N.V., a gene therapy company, announced positive top-line data from its pivotal, Phase III HOPE-B gene therapy trial of etranacogene dezaparvovec, an investigational adeno-associated virus five (AAV5)-based gene therapy for the treatment of patients with severe and moderately severe hemophilia B.
Middle East and Turkey Hemophilia Treatment Marke- Restraint
High Cost of Hemophilia Drugs
High costs of Hemophilia drugs, lack of skilled professionals, and stringent rules and regulations for product approval are the major factors that can hamper the market growth over the forecast period. For instance, in December 2021, according to the data provided by National Library of Medicine, Hemophilia A has a significant economic burden in Turkey due to costlyreplacement therapy. The major cost contributor was factor replacement therapy. With inhibitor development, the average annual cost increased more than 3-fold. In 2018, the total annual disease burden of hemophilia A in turkey was US$ 614 million, which was 1.6% of the total health expenditure in Turkey
Middle East and Turkey Hemophilia Treatment Market- Key Players
Major players operating in the Middle East and Turkey hemophilia treatment market include Takeda Pharmaceutical Company Limited., Sanofi S.A., Octapharma AG, Swedish Orphan Biovitrum AB, Baxter International Inc., Biogen Inc., Bayer AG, CSL Behring, Ferring B.V., Pfizer, Inc., Kedrion, Novo Nordisk A/S, Grifols S.A., Sangamo Therapeutics, Inc., and Spark Therapeutics, Inc.
Definition: Hemophilia A is an inherited, serious disorder in which a person’s blood does not clot properly that leads to uncontrolled and often spontaneous bleeding. Hemophilia A affects around 900,000 people worldwide, approximately 35-39% of them has severe form of the Hemophilia disorder. People with hemophilia A either lack or do not have enough clotting protein called factor VIII. In a healthy person, when a bleed occurs, factor VIII brings together the clotting factors IXa and X, this is a critical step in the formation of a blood clot to stop bleeding. Depending on the severity of disorder, people with hemophilia A can bleed frequently, especially into their joints or muscles. These bleeds causes health concern as it often cause pain, and can lead to chronic swelling, deformity, reduced mobility, and long-term joint damage.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients