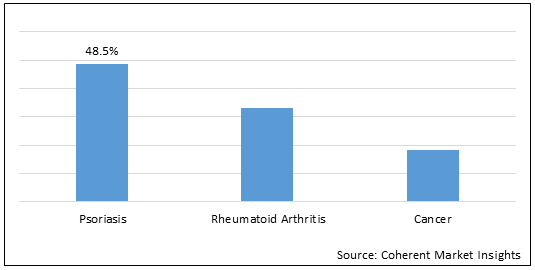
Methotrexate is a folate antagonist. Methotrexate is one of the most effective and widely used medications for treating inflammatory types of arthritis. It is also one of the safest arthritis drugs. Methotrexate drugs are indicated for the treatment of Psoriasis, Rheumatoid Arthritis. Methotrexate is also used to treat certain types of cancer including cancers that begin in the tissues that form around a fertilized egg in the uterus, breast cancer, lung cancer, certain cancers of the head and neck, certain types of lymphoma, and leukemia (cancer that begins in the white blood cells).
Methotrexate treats cancer by slowing the growth of cancer cells. Methotrexate treats psoriasis by slowing the growth of skin cells to stop scales from forming. Methotrexate may treat rheumatoid arthritis by decreasing the activity of the immune system.
Global methotrexate drugs market is estimated to be valued at US$ 590.2 million in 2022 and is expected to exhibit a CAGR of 2.6% during the forecast period (2022-2030).
Figure 1. Global Methotrexate Drugs Market Share (%), by Treatment Type, 2022
To learn more about this report, Download Free Sample
Global Methotrexate Drugs Market- Drivers
Increasing number of drug approvals by regulatory bodies is expected to drive the global methotrexate drugs market growth over the forecast period.
Increasing number of drug approvals by regulatory bodies is expected to boost the growth of global methotrexate drugs market. For instance, on March 30, 2022, NORDIC PHARMA, a SEVER Life Sciences company, announced the submission of a New Drug Submission to Health Canada for its methotrexate auto-injector, Nordimet for the treatment of severe disabling active rheumatoid arthritis (RA) and symptomatic control of severe, recalcitrant, disabling psoriasis in adults who are not adequately responsive to other forms of therapy.
Furthermore, on September 26, 2022, Eisai Co., Ltd., and nippon medac Co., Ltd., a subsidiary of medac Gesellschaft für klinische Spezialpräparate mbH announced that they have obtained manufacturing and marketing approval from the Japanese Ministry of Health, Labour and Welfare for the indication of the anti-rheumatic agent “Metoject Subcutaneous Injection 7.5mg syringe 0.15mL, 10mg syringe 0.20mL, 12.5mg syringe 0.25mL and 15mg syringe 0.30mL” (methotrexate) for the treatment of rheumatoid arthritis.
Methotrexate Drugs Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 590.2 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 2.6% | 2030 Value Projection: | US$ 735.9 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
LEO Pharma A/S, Bristol Myers Squibb, Nordic Pharma, Eli Lilly and Company, Horizon Therapeutics plc., Cumberland Pharmaceuticals Inc., Azurity Pharmaceuticals, Inc., Pfizer Inc., Hikma Pharmaceuticals PLC., and Onco Therapies Limited (Strides Arcolab Limited) |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
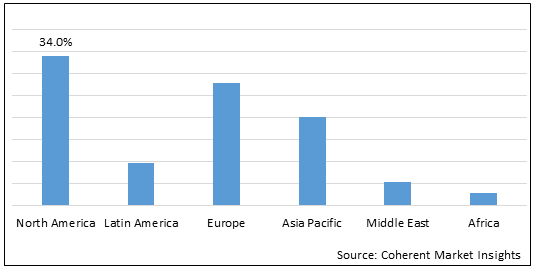
Figure 2.Global Methotrexate Drugs Market Share (%), by Region, 2022
To learn more about this report, Download Free Sample
Global Methotrexate Drugs Market- Driver
Ongoing research and development using methotrexate drugs is expected to drive the global methotrexate drugs market growth.
Key market players are focused on research and development of methotrexate drugs which is expected to drive the global methotrexate drugs market over the forecast period. For instance, Aclaris Therapeutics, Inc., a pharmaceutical company, announced that the company initiated Phase 2 trial of ATI-450 Plus Methotrexate (MTX), for the treatment of Rheumatoid Arthritis.
Global Methotrexate Drugs Market– Impact of Coronavirus (COVID-19) Pandemic
The COVID-19 pandemic has drastically affected clinical trials. Many trials have paused enrollment and researchers are facing multiple challenges associated with setting up remote visits, and performing laboratory and other study assessments.
The ability to conduct clinical trials has been significantly impacted by the COVID-19 pandemic. Many clinical trials have been halted or delayed and the enrollment of new participants is being postponed. Without important data on the safety and effectiveness of treatments from clinical trials, the arrival of new treatments in the marketplace will ultimately be delayed. In addition, patients fighting serious and life-threatening diseases may not be able to access investigational products because of suspended clinical trials.
According to NORD, the U.S. FDA issued a guidance for industry, investigators, and institutional review boards (IRBs) to inform key considerations (such as the decision to continue or suspend a clinical trial) and requirements for sponsors undertaking clinical trials during the COVID-19 outbreak. FDA’s guidance provides flexibility to sponsors to continue clinical trials where feasible and appropriate. The following are key points from the guidance:
Global Methotrexate Drugs Market: Key Developments
Market players are focused on gaining product approvals from regulatory bodies is expected to drive the growth of global methotrexate drugs market. For instance, in January 2020, Aurobindo Pharma Limited., announced that its joint venture company, Eugia Pharma Specialities Limited, has received a final approval from the U.S. Food & Drug Administration (USFDA) to manufacture and market Methotrexate tablets, 2.5 mg. Methotrexate tablets are generic version of Dava Pharmaceuticals’ Rheumatrex Tablets.
Global Methotrexate Drugs Market: Restraint
Side effects associated to Methotrexate drugs, is expected to hinder the growth of global methotrexate drugs market. Side effects associated with methotrexate drugs are:
Key Players
Major players operating in the global methotrexate drugs market include LEO Pharma A/S, Bristol Myers Squibb, Nordic Pharma, Eli Lilly and Company, Horizon Therapeutics plc., Cumberland Pharmaceuticals Inc., Azurity Pharmaceuticals, Inc., Pfizer Inc., Hikma Pharmaceuticals PLC., and Onco Therapies Limited (Strides Arcolab Limited).
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients