The global metastatic melanoma therapeutics market is estimated to be valued at USD 8.00 Bn in 2025 and is expected to reach USD 18.02 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 12.3% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The metastatic melanoma therapeutics market is expected to witness a positive trend over the forecast period due to increasing incidences of melanoma cases globally. According to some reports, over 200,000 new cases of melanoma were reported in 2020. This high prevalence has led to increased research and development activities in terms of new drug development and approval. Many pharmaceutical companies are focusing on developing novel treatments for metastatic melanoma such as targeted therapies and immunotherapies. This is anticipated to provide potential growth opportunities for market players operating in this domain.
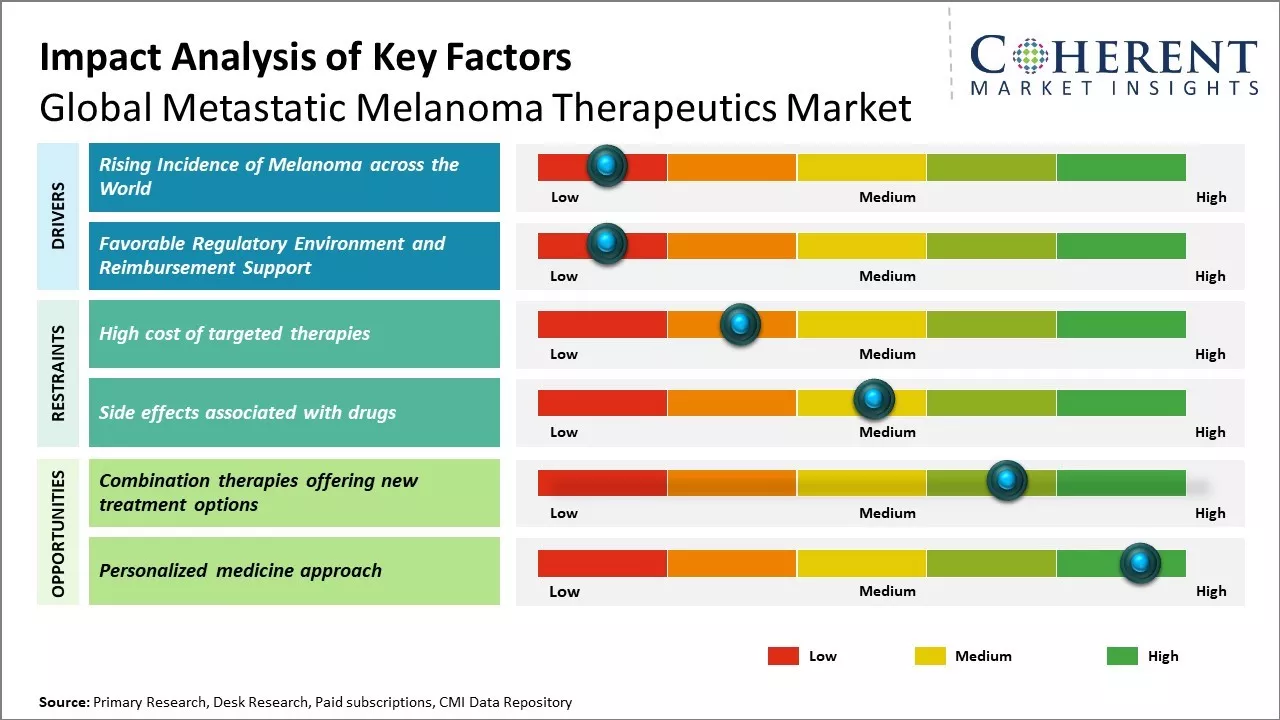
Rising Incidence of Melanoma across the World
The global incidence of melanoma has been rising rapidly in the last few decades. While melanoma accounts for only 1% of skin cancer cases, it causes a majority of skin cancer deaths. The increasing prevalence of indoor tanning and lack of protection from sun exposure has contributed significantly to the rising melanoma burden worldwide. Regions with fair-skinned populations like North America, Australia, and Europe have seen the highest increases. However, melanoma rates are growing in many other parts as well, especially in developing countries where awareness has been traditionally low. Experts forecast that melanoma could become even more common in the future if appropriate sun protection measures are not taken on a large scale. More people being diagnosed with melanoma naturally leads to greater demand for effective therapeutics. Compared to other cancer types, treatment options for advanced or metastatic melanoma used to be very limited until recent years. Earlier therapeutics provided only modest benefits and severe toxicities were common. The introduction of immunotherapy drugs like checkpoint inhibitors has revolutionized melanoma treatment. These new treatment classes have shown unprecedented and durable responses. However, they still do not work for every patient and drug resistance often develops. This further stimulates the development of new and improved treatment approaches. For instance, in April 2025, data from the World Health Organization (WHO) highlights a rising incidence of cutaneous malignant melanoma (CMM) among adults aged 45 to 64 years in many countries worldwide, except Australia. Despite this increasing prevalence, CMM-specific mortality has shown a decrease in recent years, particularly in high-income countries. Studies, including those from the University of Milan, Italy, analyzing WHO data from 1980 to 2019, reveal that while incidence rates have been consistently rising, CMM-related mortality rates surged in the 1990s but have since stabilized in most high-income countries. For younger adults aged 20 to 44 years, between 2015 and 2019, age-standardized mortality rates (ASMRs) varied across different countries, with figures ranging from 0.10 to 1.11 per 100,000 for men and from 0.11 to 0.81 per 100,000 for women.
Get actionable strategies to beat competition: Download Free Sample
Favorable Regulatory Environment and Reimbursement Support
Regulators in the major pharma markets have adopted an accommodative stance to fast-track approval of innovative melanoma drugs. They acknowledge the traditional lack of good treatment options and high unmet need. Developers of targeted therapies and immunotherapies have greatly benefitted from this supportive regulatory environment. For example, the U.S. FDA and EMA, European Medicines Agency have approved several new drug applications based on early clinical trial data, omitting the need for phase III studies in some cases. The priority review and approval designations help reach patients sooner. This encourages continued research and commercialization of new therapeutic entities. On the reimbursement front, private and government payers seem willing to cover the high costs of modern melanoma medicines. The improved outcomes and survival benefits demonstrated in clinical testing help justify good reimbursement decisions. Coverage policies also consider the economic burden of advanced melanoma and high total healthcare spending in the absence of effective treatments. Higher drug prices are considered acceptable due to the benefits of new mechanisms and smaller subsequent healthcare resource use.
Key Takeaways from Analyst:
The global metastatic melanoma therapeutics market appears promising in the coming years. Growing prevalence of melanoma and availability of various treatment options are expected to drive the market growth. Furthermore, increasing adoption of targeted therapies and availability of late-stage pipeline drugs will provide opportunities for market growth.
North America currently dominates the market and is expected to continue its dominance over the forecast period. High healthcare expenditure and availability of advanced treatment facilities in the U.S. and Canada are some factors responsible for North America's large share.
However, Asia Pacific is likely to witness the highest growth rate led by China and India. This can be attributed to rising healthcare expenditure, growing awareness regarding melanoma, and increasing customer base.
On the other hand, lack of awareness about melanoma in developing regions may hamper the market growth. Moreover, high treatment cost associated with therapeutics such as immunotherapies and targeted therapies can negatively impact the market growth, especially in price-sensitive regions.
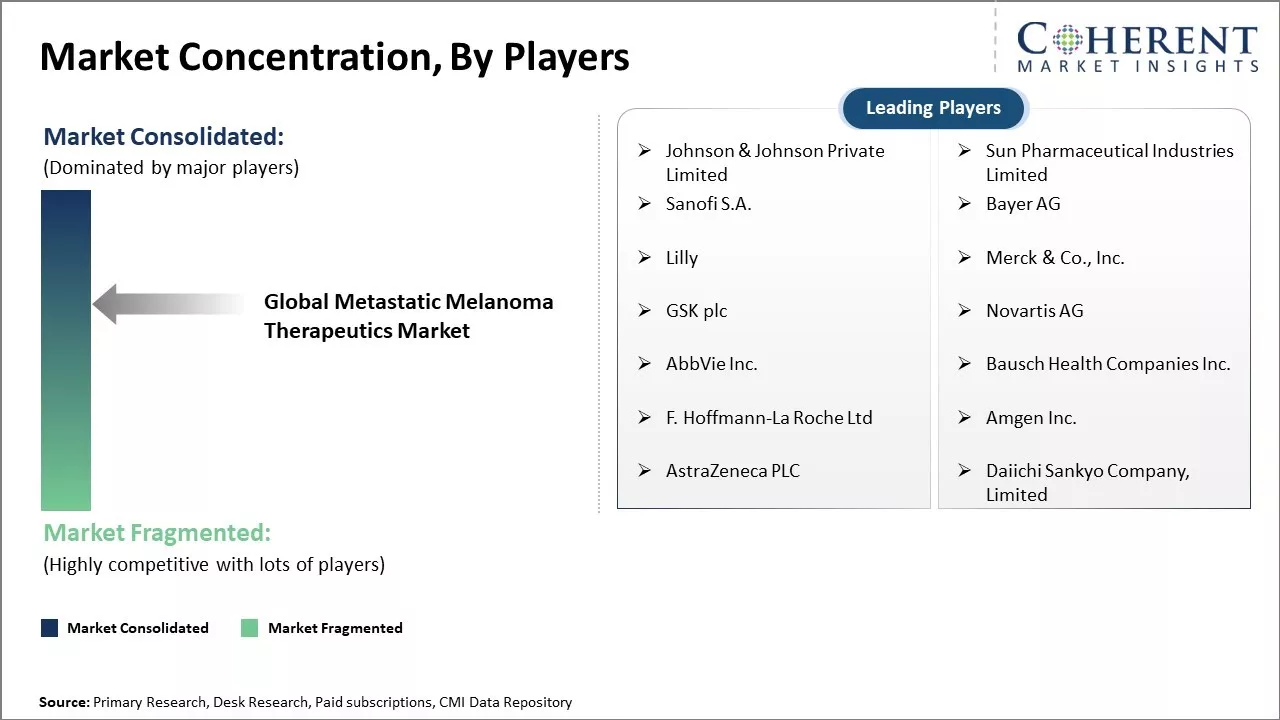
The market is highly competitive with major players continuously focusing on new product development and commercialization. Successful research collaborations between key players are further assisting clinical development. Some companies are also evaluating combination therapies to gain a competitive edge in the market.
Market Challenges: High cost of targeted therapies
The high cost of targeted therapies continues to pose significant challenges for the growth of the global metastatic melanoma therapeutics market. While targeted therapies have significantly improved survival rates compared to traditional chemotherapy, their prices remain exceedingly high for many patients. For instance, immunotherapies like Opdivo and Keytruda which have revolutionized melanoma treatment cost upwards of US$ 150,000 for a year's worth of treatment. Similarly, BRAF and MEK inhibitor drug combinations that are the standard first-line targeted therapy for BRAF mutation-positive melanoma have average list prices of over US$100,000 per year. With many patients requiring treatment for several years, the total drug costs easily exceed hundreds of thousands of dollars. This financial toxicity associated with prolonged therapy puts targeted drugs out of reach for many patients who lack comprehensive health insurance or rely on public insurance programs.
Market Opportunities: Combination therapies offering new treatment options
Combination therapies have the potential to bring revolutionary changes in the treatment of metastatic melanoma. Using multiple drugs that work through different pathways and mechanisms has significant advantages over single agent therapies. Combinations are able to target diverse molecular alterations and overcome resistance mechanisms. This makes them less prone to the development of resistance by cancer cells. Recent data from clinical trials shows promising results for combination regimens in metastatic melanoma. For example, the combination of targeted therapies like BRAF and MEK inhibitors has shown improved progression free survival and overall survival over BRAF inhibitors alone. According to the American Cancer Society, the 5-year survival rate for stage 4 melanoma patients increased to 26% for 2014-2020 from 6% in 1975-1977, largely attributed to advancements in targeted therapies and immunotherapy. The success of immunotherapy drugs like anti-PD-1 antibodies used in combination with other agents is also spurring better outcomes. As combination treatments continue demonstrating better efficacy, the trend of their increased usage is expected to dominate the therapeutic landscape in the coming years. Manufacturers are investing heavily in developing novel combination drug regimens through strategic collaborations.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
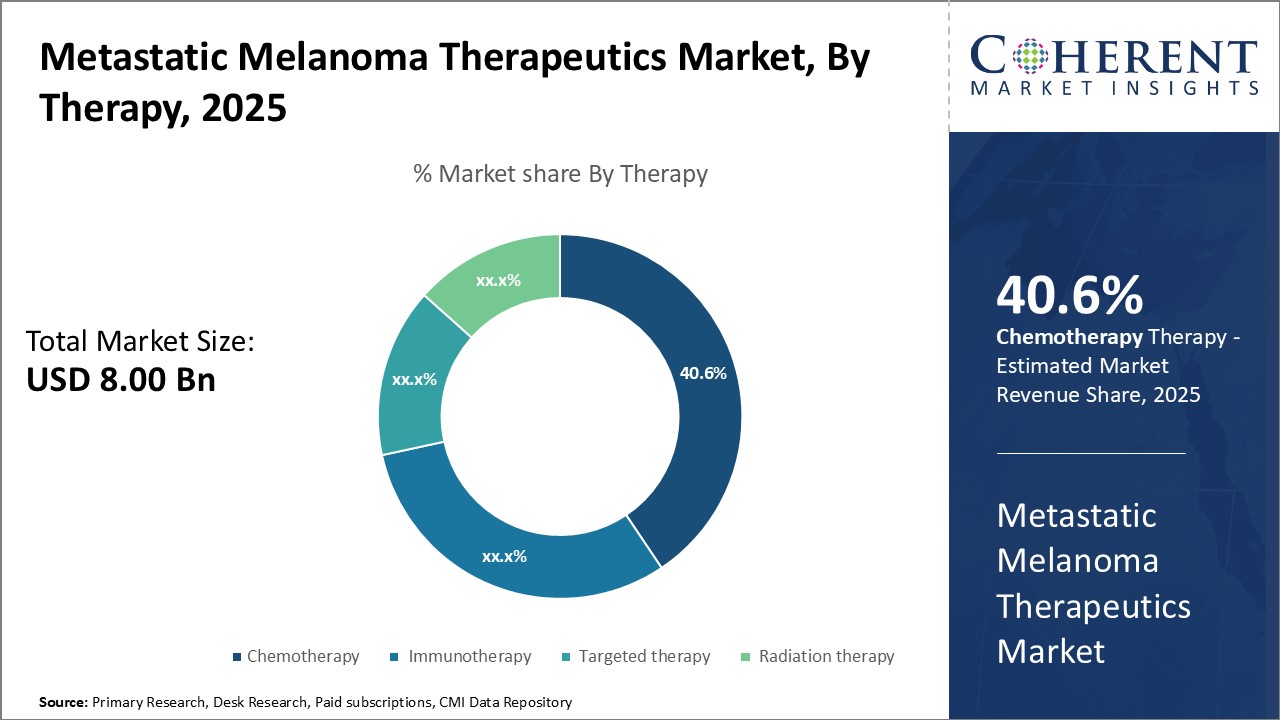
By Therapy - Rising Prevalence of Advanced Stage Cancer Boosts the Chemotherapy Demand
In terms of therapy, chemotherapy is expected to contribute 40.6% of the market share in 2025 owing to its widespread adoption for treating advanced stage metastatic melanoma. Chemotherapy utilizes cytotoxic anti-cancer drugs to eliminate rapidly dividing malignant cells by interfering with cell division and growth. The growing prevalence of Stage IV metastatic melanoma across populations spurs the use of chemotherapy as an important line of defense. Patients diagnosed at later stages are often recommended chemotherapy in combination with other innovative therapies to increase treatment effectiveness against aggressive tumor growth and metastasis. Moreover, chemotherapy remains a backbone treatment option when immunotherapy and targeted therapies are ineffective or inappropriate for certain patients. Its well-established mechanisms for fighting cancer cells provide a reliable option for advanced disease management.
By Stages - Unmet Needs in Late-stage Disease Propel Stage IV Dominance
In terms of stages, Stage IV is expected to contribute 25.5% of the market share in 2025 owing to significant unmet medical needs in metastatic melanoma diagnosed at later stages. Stage IV entails melanoma that has spread from the primary site to other organs such as the lungs, brain, or bones. This advanced level of disease poses far greater challenges due to widespread tumor presence across the body. Currently available treatments are often inadequate to achieve long-term remission or cure at Stage IV, driving ongoing research into more potent therapies and combinations. Additionally, patients diagnosed at Stage IV tend to have already received previous treatments that failed, necessitating subsequent lines of specialized therapies tailored for their individual cancer profiles. These factors reinforce the large patient segment and commercial opportunities surrounding Stage IV metastatic melanoma management.
By End User - Expertise and Infrastructure Make Hospitals Hub of Treatment
In terms of end user, hospitals is expected to contribute 40.62% of the market share in 2025 due to their prominence as full-service medical facilities. The complex nature of metastatic melanoma diagnosis and management demands a high level of multidisciplinary expertise available at tertiary care settings. Hospitals house specialized oncology departments, diagnostic laboratories, interventional radiology suites and other infrastructure integral to coordinating multimodal treatment plans involving multiple therapists such as medical and radiation oncologists, surgeons and dermatologists. They also offer round-the-clock monitoring and care especially vital during intensive procedures or complications management. This comprehensive service portfolio, together with established clinical trials participation, cements the role of hospitals as premier treatment access points for advanced melanoma patients worldwide seeking holistic and tailored care in a single location.
Need a Different Region or Segment? Download Free Sample
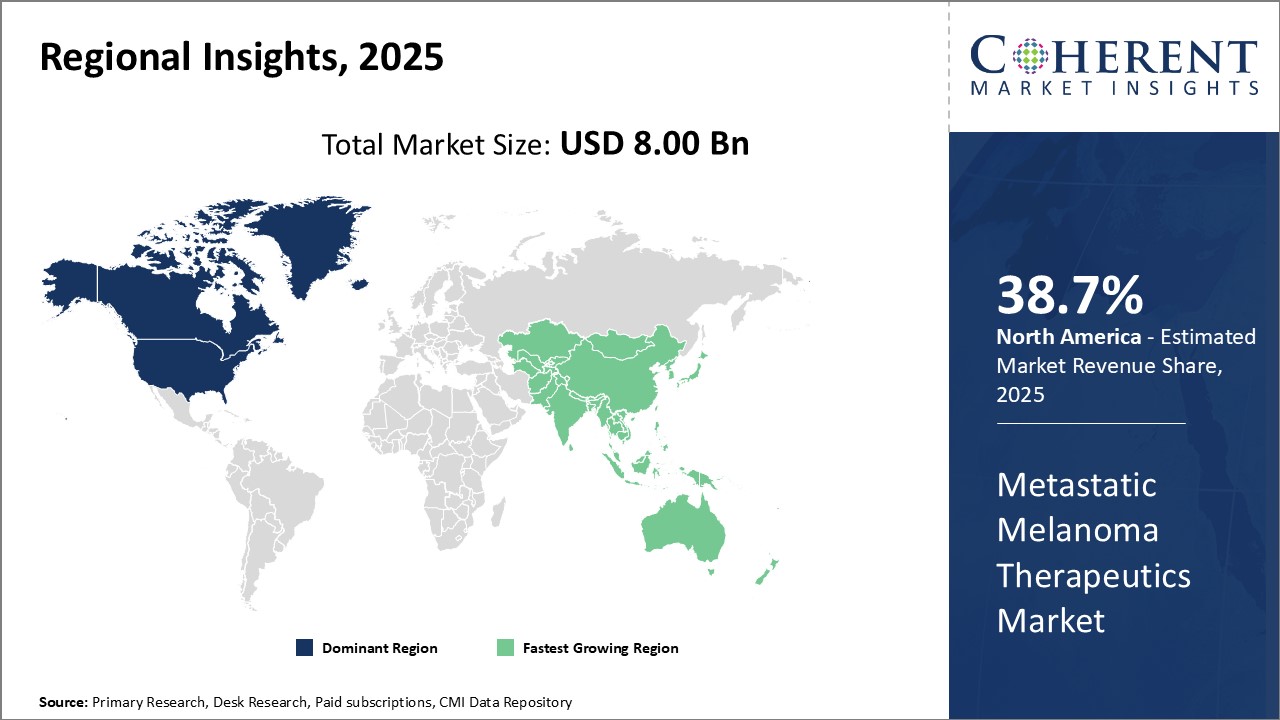
North America has firmly established itself as the dominant regional market. The region is expected to account for a market share of 38.7% in 2025 for metastatic melanoma therapeutics. This can mainly be attributed to the strong presence and growth of pharmaceutical companies in the region, especially in the U.S. Major players such as Bristol-Myers Squibb, Merck, Roche, and Pfizer have their headquarters in the U.S. and invest heavily in R&D for innovative therapeutics to treat melanoma and other cancers. Furthermore, the U.S. FDA swiftly approves new drugs that show promising results in clinical trials. As a result, patients in the region have early access to the most advanced treatment options. The high healthcare spending by governments as well as individuals also ensures that people are able to afford high-priced targeted therapies and immunotherapies.
The Asia Pacific region, on the other hand, represents the fastest growing market for metastatic melanoma therapeutics. Countries such as China, Japan and South Korea have a large patient population and a rising incidence rate of melanoma due to increased exposure to UV radiation and growing awareness. At the same time, improving accessibility and infrastructure is allowing more people to be diagnosed and seek treatment. International drug makers see immense potential for future growth and are actively engaged in partnerships with local players for manufacturing and distribution of drugs. They are also conducting clinical trials to generate regional clinical data packages required for regulatory approval. Several Asian governments provide subsidies to make expensive therapies affordable. These factors are expected to fuel the stronger adoption of novel therapies and robust expansion of the regional metastatic melanoma therapeutics market over the coming years.
Metastatic Melanoma Therapeutics Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 8.00 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 12.3% | 2032 Value Projection: | USD 18.02 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Johnson & Johnson Private Limited, Sun Pharmaceutical Industries Limited, Sanofi S.A., Bayer AG , Lilly, Merck & Co., Inc., GSK plc , Novartis AG, AbbVie Inc., Bausch Health Companies Inc., F. Hoffmann-La Roche Ltd, Amgen Inc., AstraZeneca PLC, and Daiichi Sankyo Company, Limited |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: The global metastatic melanoma therapeutics market involves developing and providing treatments that specifically target metastatic melanoma. This includes immunotherapy drugs, targeted therapies, and other therapeutics that can help fight melanoma cancer once it has spread from the skin to other parts of the body like the lungs, brain, bones or liver. The goal is to develop more effective and personalized treatments to improve survival rates for patients suffering from late-stage metastatic melanoma.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients