Global meningococcal vaccines market is estimated to be valued at USD 4.53 Bn in 2025 and is expected to reach USD 9.05 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 10.4% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
Rising prevalence of meningococcal meningitis globally can boost demand for effective vaccination and immunization against the disease. Government initiatives for immunization and availability of expanded vaccine coverage under national immunization programs in various countries can also drive the market growth.
The market is witnessing trends like new product launches targeted at providing protection against additional serogroups of N. Meningitidis. Major manufacturers are investing in development of vaccines for serogroup B meningococcal disease with goal to provide broader protection. Moreover, combination vaccines providing protection against multiple serogroups in single dose are also gaining popularity. This is expected to boost vaccination uptake and compliance.
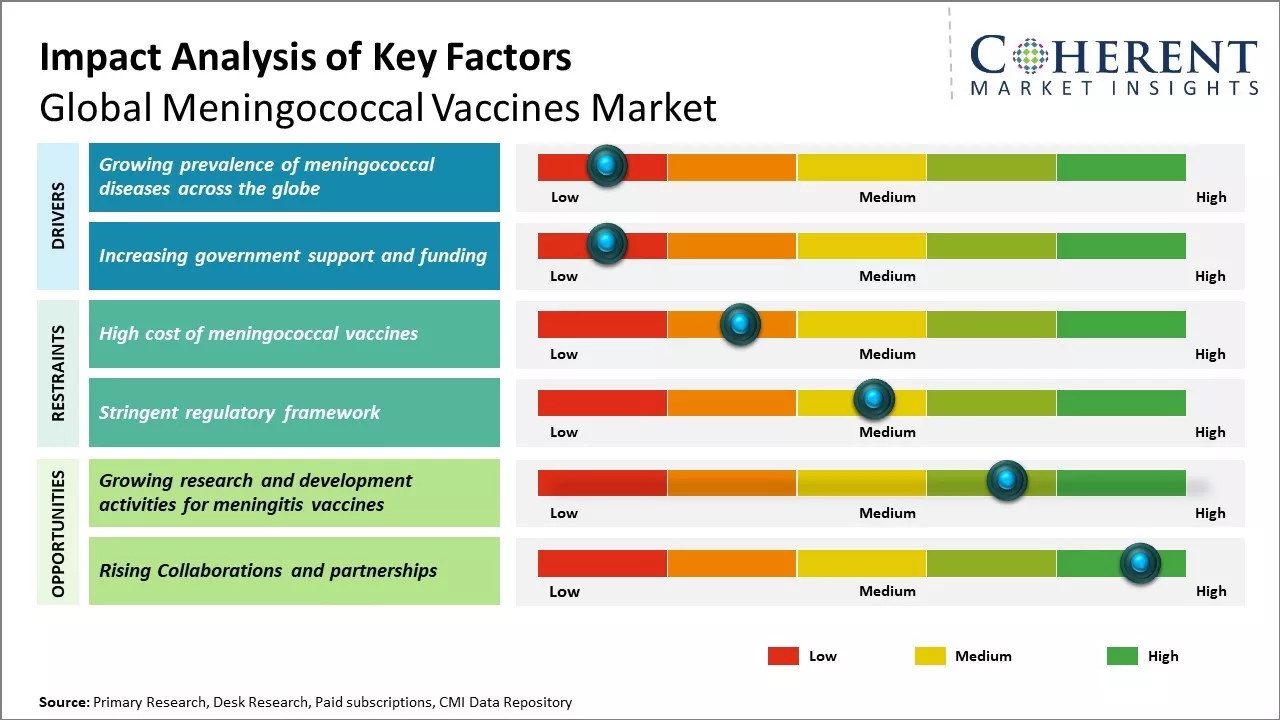
Growing prevalence of meningococcal diseases across the globe
Growing prevalence of meningococcal diseases across the world can drive the market growth. According to the World Health Organization (WHO), meningococcal meningitis causes over 1.2 million cases and approximately 135,000 deaths worldwide annually. The bacterium Neisseria meningitidis can cause severe sepsis and meningitis with high mortality rates, if not treated promptly. According to the data from WHO, the highest rates of meningococcal diseases are reported from sub-Saharan Africa, with the 'Meningitis Belt' extending from Senegal to Ethiopia being the most affected region. Countries in this belt experience regular epidemics, with over 800,000 cases and over 7500 deaths reported in the 2020-2021 meningitis season. However, other parts of world like Europe, North America and Brazil are also witnessing increasing incidence of different meningococcal serogroups, thus, leading to growing disease burden. This includes recent serogroup B epidemics in the U.K and serogroup W outbreaks in Latin America. Rising global prevalence of meningococcal diseases has made vaccination a public health priority. All the major vaccine bodies including WHO now recommend inclusion of meningococcal vaccines in routine childhood immunization programs along with catch-up campaigns for high-risk groups. For instance, on March 28, 2025, The Centers for Disease Control and Prevention issued a warning about rise in serious meningococcal disease in the U.S., mainly caused by a type called Neisseria meningitidis serogroup Y. Last year, there were 422 cases reported, the highest since 2014, and as of March 25, 2025, there have been 143 cases reported. A specific strain, ST-1466, is behind most of these cases, affecting mostly people aged 30-60, Black or African American individuals, and those with HIV. This strain often causes blood infections instead of meningitis, and the death rate has risen to 18%. Healthcare providers are being urged to be vigilant, especially among high-risk groups, and ensure people at risk, including those with HIV, are vaccinated on time.
Get actionable strategies to beat competition: Download Free Sample
Increasing government support and funding
Growing governmental support and funding for development of expanded meningococcal vaccination programs globally can drive the market growth. Several governments recognize that widespread vaccination can help prevent or minimize the impacts of meningococcal disease outbreaks, which puts significant burden on the public health system. This encourages pharmaceutical companies to invest more resources into research and development of improved vaccines with broader coverage. Regulatory bodies have also streamlined approval processes for new meningococcal vaccines to facilitate their accessibility. Many national immunization technical advisory groups now recommend inclusion of additional serogroups in routine infant vaccination schedules based on emerging disease trends. This is translating to higher procurement of multivalent conjugate vaccines by national immunization programs. International organizations like WHO also supports underdeveloped countries through funding and technology transfers to introduce vaccines for controlling epidemic prone serogroups as part of their national immunization programs.
Key Takeaways from Analyst:
Global meningococcal vaccines market growth is driven by factors such as increasing government initiatives towards immunization programs worldwide and growing awareness about meningococcal disease. Rising healthcare spending in developing nations also increases access to these life-saving vaccines. However, strict regulatory landscape and long approval timelines for new products can hamper the market growth.
North America dominates the market due to high awareness and strong reimbursement structure in the region. Asia Pacific is poised to witness the fastest growth owing to improving economic conditions and growing focus of pharmaceutical companies in the region. Within Asia Pacific, China and India will emerge as major revenue generators for meningococcal vaccine manufacturers.
Vaccine companies need to focus on expanding their geographic footprint beyond developed markets. Partnerships with local pharmaceutical players can help increase access in higher growth emerging economies. Introducing combination meningitis vaccines that protect against multiple serogroups can offer market growth opportunities. Achieving World Health Organization prequalification status for vaccines will allow companies to target global procurement agencies.
Market Challenges: High cost of meningococcal vaccines
The high cost of meningococcal vaccines can hamper the global meningococcal vaccines market growth. Developing advanced vaccines requires huge investments in research and clinical trials over many years. Vaccine manufacturers have to recover these huge research and development costs through the prices of the vaccines. However, pricing vaccines at elevated levels makes them unaffordable for large populations in low and middle-income countries. Vaccines for meningococcal disease, such as serogroup B meningococcal vaccines, typically have a list price in developed markets between US$ 150 to US$ 250 per dose. This is significantly higher than most traditional childhood vaccines which cost under US$ 50 per dose. The high cost becomes a major barrier especially in developing nations where a vast majority cannot afford or have access to subsidized vaccination programs for such expensive vaccines. Most countries spend only US$ 1-2 per person annually on immunization, which is not enough to cover advanced meningococcal vaccines through national programs.
Market Opportunities: Growing research and development activities for meningitis vaccines
Increasing R&D activities for developing meningitis vaccines can offer opportunity for the global meningococcal vaccines market growth. Meningitis remains a major public health challenge worldwide, especially in developing countries. According to WHO, meningococcal meningitis leads to approximately 250,000 infections and 26,000 deaths annually across Africa's meningitis belt alone. The high disease burden has prompted extensive research efforts for effective vaccination programs. Pharmaceutical companies and research institutions are actively working on improving the existing vaccine technologies as well as developing new and advanced vaccines. Several candidates are in clinical trial phases for immunization against the various serogroups of Neisseria meningitidis bacteria. This includes vaccines promising broader protection coverage as well as single-dose vaccines for ease of use. For example, Pfizer and the Bill and Melinda Gates Foundation collaborated to develop an affordable meningococcal conjugate vaccine for use in Africa and Asia. Such initiatives have helped accelerate the R&D of vaccines tailored for resource-poor regions disproportionately affected by meningitis outbreaks.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
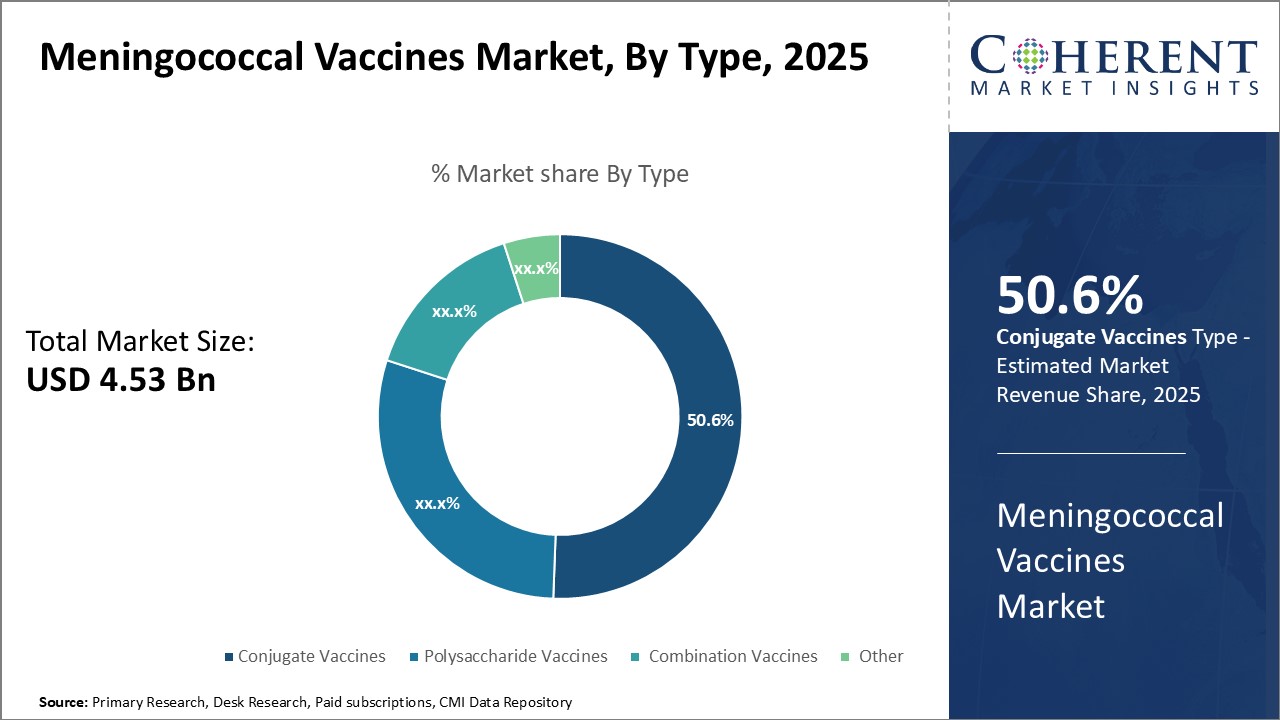
Insights, By Type - Innovation and Advancement Boosts Adoption of Conjugate Vaccines
In terms of type, conjugate vaccines segment is estimated to contribute the highest market share of 50.6% in 2025, owing to continuous innovation and advancements in their formulations. Conjugate vaccines provide long lasting protection and immune memory against multiple serogroups of meningococcal bacteria as compared to polysaccharide vaccines. These generate both antibodies and cellular immune response essential for swift activation of immune cells against future infections. Recent innovations involve introduction of new generation tetravalent and pentavalent conjugate vaccines covering additional serogroups in a single dose. Their enhanced immunogenic profiles have enabled protection from a wider range of strains. Regulatory approvals and recommendations from public health organizations for their widespread use have further boosted their adoption. Strong research pipeline exploring novel carrier proteins and conjugation chemistries promise continued upgrades to conjugate vaccines.
Insights, By Sales Channel - Public Health Initiatives Support Public Segment
In terms of sales channel, public segment is estimated to contribute the highest market share of 60.10 % in 2025, due to nationwide immunization programs. Governments prioritize incorporation of affordable conjugate vaccines into national vaccination schedules based on disease epidemiology and recommendations from WHO. Mass public vaccination drives organized through primary health centers help to achieve high coverage goals. Subsidies on publicly procured vaccine stocks make them accessible for vulnerable population groups. Strengthening of public health infrastructure over the years has enhanced distribution network reaching remote areas. Advisory bodies promoting benefits of routine immunization to public also boosts demand.
Insights, By Age Group - Risk Exposure Drives Uptake in Children and Adults
In terms of age group, children and adults (2 years & above) segment is estimated to contribute the highest market share of 70.5% in 2025 due to higher probability of exposure and risk of disease. Growing childhood population and extended life expectancy have raised the susceptible population base over time. Younger children, adolescents and adults living in densely populated environments or university campuses are at a higher risk of acquiring the infection. Occupational hazards for military personnel and laboratory workers further boost uptake in this group. Continuous awareness about effective prevention through vaccination helps parents and individuals make informed choices.
Need a Different Region or Segment? Download Free Sample
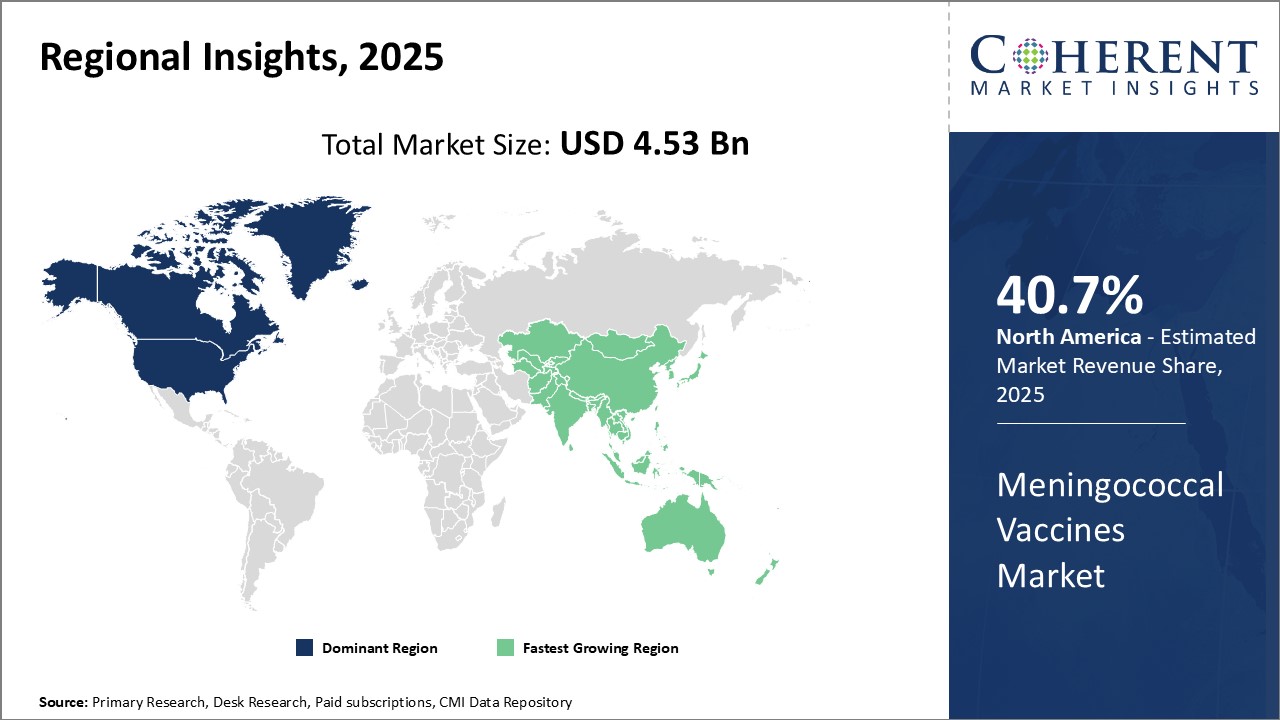
North America dominates the global meningococcal vaccines market with an estimated market share of 40.2 % in 2025, owing to high awareness levels regarding various meningococcal diseases and their treatment options. The region has a well-established healthcare sector backed by solid healthcare infrastructure and increasing healthcare spending. This allows for easy access to advanced treatment solutions like meningococcal vaccines.
Stringent regulations have ensured the development and adoption of high-quality vaccines. Presence of top vaccine manufacturers has made the region self-sufficient in terms of production. These companies invest heavily in R&D to develop newer and improved vaccine formulations. Favorable reimbursement policies further drive the market growth in North America.
Asia Pacific is emerging as the fastest growing regional market. Factors such as rising living standards, growing medical tourism and increasing healthcare investments are driving the market growth. Burgeoning population makes these countries more prone to infectious disease outbreaks, raising need for preventive immunization drives.
Rapid economic development in countries like China and India is positively impacting their healthcare sectors. Both domestic players as well as global companies are keenly looking to tap growth opportunities by establishing production and distribution networks in Asia. Awareness building campaigns by governments and global health organizations boosts demand for vaccines.
Meningococcal Vaccines Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 4.53 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.4% | 2032 Value Projection: | USD 9.05 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
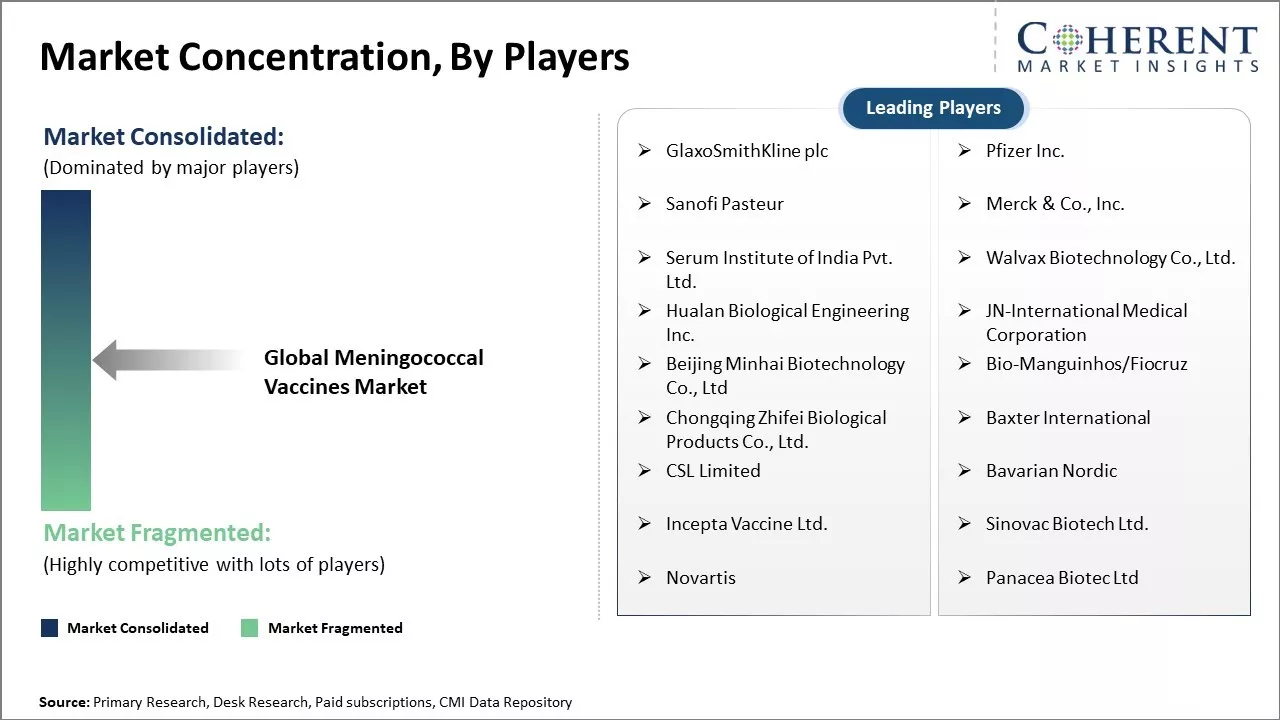
GlaxoSmithKline plc, Pfizer Inc., Sanofi Pasteur, Merck & Co., Inc., Serum Institute of India Pvt. Ltd., Walvax Biotechnology Co., Ltd., Hualan Biological Engineering Inc., JN-International Medical Corporation, Beijing Minhai Biotechnology Co., Ltd, Bio-Manguinhos/Fiocruz, Chongqing Zhifei Biological Products Co., Ltd., Baxter International , CSL Limited, Bavarian Nordic, Incepta Vaccine Ltd., Sinovac Biotech Ltd., Novartis, Panacea Biotec Ltd |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: Global meningococcal vaccines market consists of manufacturers and stakeholders who develop and provide various types of vaccines to protect against meningococcal disease. This market involves the production, licensing, distribution and sale of different meningococcal vaccines that help prevent infections caused by Neisseria meningitidis bacteria. The vaccines target the most common serogroups that cause epidemics worldwide. The overall aim of this market is to make effective meningococcal vaccines available globally to reduce the public health burden of this life-threatening bacterial infection.
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients