MENA Biologics & Biosimilars Market is estimated to be valued at USD 549.3 Mn in 2025 and is expected to reach USD 752.5 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 4.6% from 2025 to 2032.
Analysts’ Views on MENA Biologics & Biosimilars Market:
A biosimilar is exactly what its name implies. It is a biologic that is 'similar' to another biologic medicine (known as a reference product), which is already licensed by the U.S. Food and Drug Administration (FDA). Biosimilars are highly similar to the reference product in terms of safety, purity, and potency, however, it may have minor differences in clinically inactive components. In approving biosimilars, the U.S. FDA may require that manufacturers conduct a clinical study (or studies) sufficient to establish safety, purity for which the reference product is licensed. The main advantage of biosimilars is its cost. Biologics and biosimilars are specialty drugs that are often on the highest tiers of health insurance plan formularies, that means patients usually have to pay high coinsurance rates for coverage.
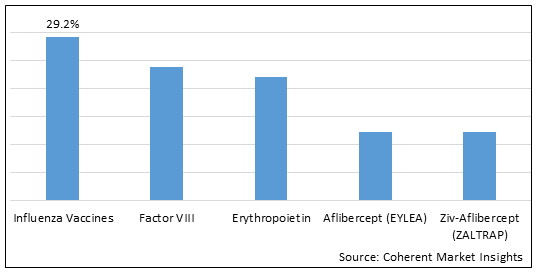
Figure 1. MENA Biologics & Biosimilars Market Share (%), By Product Type, 2025
To learn more about this report, Download Free Sample
MENA Biologics & Biosimilars Market– Drivers
Key players are focusing on facility expansion
The key players in the region are focusing on facility expansion, which is expected to drive the growth of the market in the near future. For instance, on June 21, 2023, the Public Investment Fund (PIF), Saudi Arabia’s global investment organization, announced that it had launched Lifera, a commercial-scale contract development and manufacturing organization (CDMO). The CDMO will enable the growth of the local bio/pharmaceutical industry, strengthen national resilience, and support Saudi Arabia’s position as a global pharmaceutical manufacturing destination.
Figure 2. MENA Biologics & Biosimilars Market Share (%), By Region/Country, 2025
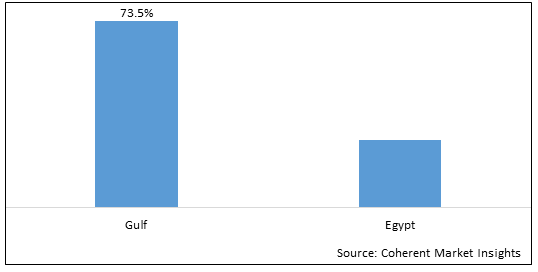
To learn more about this report, Download Free Sample
MENA Biologics & Biosimilars Market- Regional Analysis
Among region, the Gulf region is estimated to hold a dominant position in the MENA biologics & biosimilars market over the forecast period owing to increasing government initiatives for vaccination campaigns that boosts thesegment growth. For instance, on October 16, 2020, the Ministry of Health (Kuwait) launched a seasonal vaccination campaign for infectious diseases such as seasonal influenza and pneumococcal pneumonia.
MENA Biologics & Biosimilars Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 549.3 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 4.6% | 2032 Value Projection: | USD 752.5 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Pfizer, Inc., F. Hoffmann-La Roche Ltd, Takeda Pharmaceutical Company Limited, Regeneron Pharmaceuticals Inc., Sanofi, Amgen Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
MENA Biologics & Biosimilars Market– Impact of Coronavirus (COVID-19) Pandemic
The COVID-19 pandemic and lockdown in various countries across the globe have positively impacted the financial status of businesses across all sectors. The biotechnology sector is one such sector that has been heavily impacted by the pandemic.
Factors such as investments, research, and development of novel medicines targeting COVID-19, as well as funding by various companies operating in the MENA are expected to drive the MENA Biologics & Biosimilars Market growth during the COVID-19 pandemic. For instance, in March 2020, the government of Saudi Arabia announced that it had donated US$ 10 million as financial support to the World Health Organization (WHO). The money is allocated for research and development of vaccines, diagnostics, and therapeutics for disease treatment. In April 2020, the Coalition for Epidemic Preparedness and Innovations (CEPI) and Gavi, the Vaccine Alliance, announced that Group 20 of Kingdom of Saudi Arabia granted US$ 150 million for the development of the COVID-19 vaccine for COVID-19 infection. CEPI is working with Curevac, Inovio Pharmaceuticals, Moderna, Novavax, The University of Hong Kong, The University of Oxford, The University of Queensland, and a consortium led by Institut Pasteur for COVID-19 vaccine development projects.
MENA Biologics & Biosimilars Market Segmentation:
The MENA biologics & biosimilars market is segmented by product type, by therapeutic application, and by region.
MENA Biologics & Biosimilars Market- Cross Sectional Analysis:
Among region/country, Product type segment in Gulf region is expected to dominate the market owing to the market players are focused on the increasing inorganic activities such as agreements, which are expected to drive market growth in the region. For instance, on April 4, 2022, Intas Pharmaceuticals Ltd. (Intas), a pharmaceutical company, signed an exclusive license and supply agreement with Axantia Holding (Axantia), a pharmaceutical company in the Middle East operating through its pharmaceutical subsidiaries, Pharma International Pharmaceutical & Chemical Manufacturing Co. Ltd. and Med City Pharmaceutical Industries, for Ranibizumab (a biosimilar of Lucentis). Under the terms of the agreement, Axantia has marketing authorization, and can commercialize Ranibizumab in certain territories, including Saudi Arabia, Jordan, Iraq, Lebanon, and GCC countries.
MENA Biologics & Biosimilars Market: Key Developments
MENA Biologics & Biosimilars Market: Key Trends
MENA Biologics & Biosimilars Market: Restraint
Compliance with stringent regulatory guidelines
MENA Biologics & Biosimilars Market - Key Players
Major players operating in the MENA biologics & biosimilars market include Pfizer, Inc., F. Hoffmann-La Roche Ltd, Takeda Pharmaceutical Company Limited, Regeneron Pharmaceuticals Inc., Sanofi, and Amgen Inc.
Definition: Biologics include medicines that generally come from living organisms, which can include animal cells and microorganisms such as yeast and bacteria. Biologics (including insulin) generally come from living organisms, so nature varies and structures are generally more complex. A biosimilar is a biologic that is highly similar to another biologic that is already regulatory-approved (known as the original biologic). It is both normal and expected for both biosimilars and original biologics to have minor differences between batches of the same medication. This means that biologics cannot be copied exactly, and that is why biosimilars are not identical to original biologic.
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients