Global medical implant sterile packaging market IS estimated to be valued at USD 2.39 Bn in 2025 and is expected to reach USD 4.19 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 8.3% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
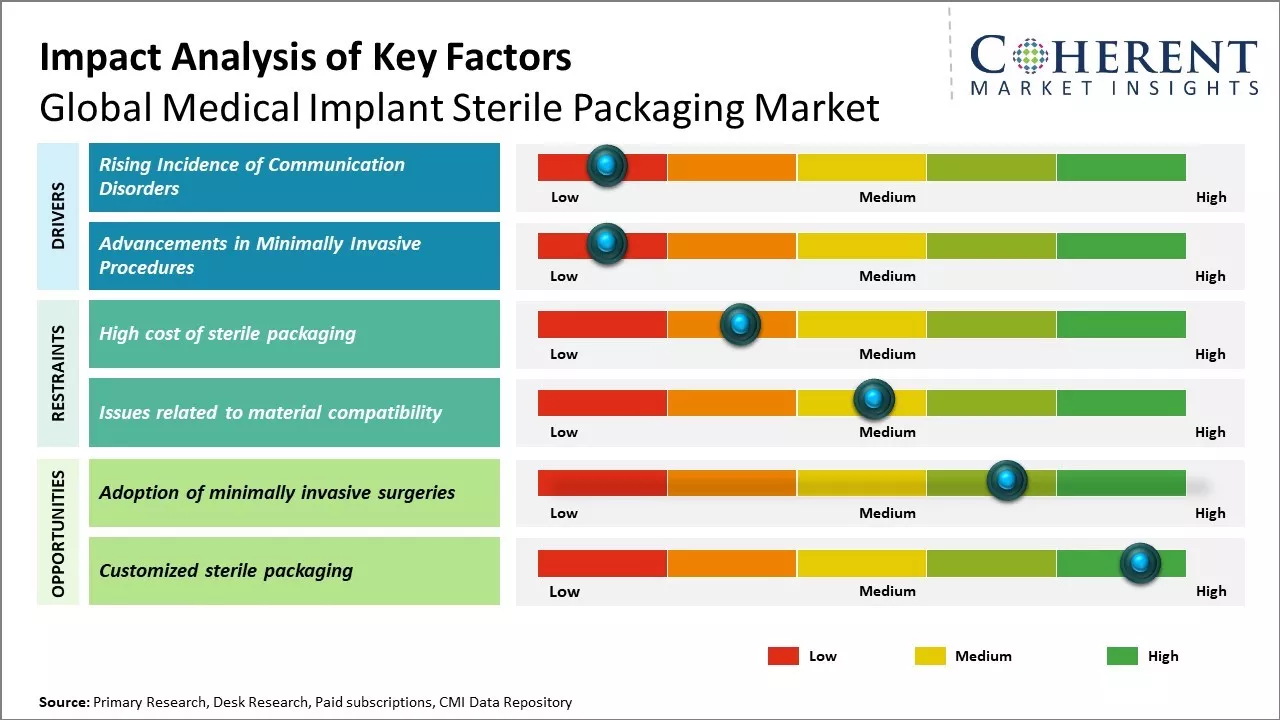
The market growth is driven by factors such as increasing prevalence of chronic diseases, rising volume of surgeries performed worldwide, and growing demand for minimally invasive procedures. Stringent regulatory guidelines for sterile packaging and growing awareness regarding benefits of sterile packaging of medical implants can boost demand. Developing healthcare infrastructure in emerging nations and rapid technological advancements in packaging solutions can drive the market growth. However, high costs associated with sterile packaging and concerns regarding packaging integrity can hinder the market expansion during the forecast period.
Rising Aging Population
Due to aging population, there is rising prevalence of age-related chronic diseases and conditions that require surgical intervention and medical implants. Older adults often suffer from arthritis, orthopedic implants, cardiovascular disease and other implants. For instance, knee replacements are one of the most common surgical procedures performed on the geriatric population suffering from osteoarthritis or other joint diseases. With emerging economies witnessing increase in life expectancy, there has been huge demand for quality healthcare and ongoing management of older patients. Growing dependency ratio and expanding base for age-related targeted therapies that can substantially improve quality of life for older adults can boost demand for implantable medical devices. Thus, rising number of surgical procedures performed on the geriatric population can boost the need for sterile packaged implants and components. For instance, according to World Health Organization Data, the global population aged 60 years and older was 1 billion in 2019, and is expected to rise to 1.4 billion by 2030 and 2.1 billion by 2050. This demographic shift boosts demand for sterile packaging in the pharmaceutical industry due to increased medical product consumption among aging populations. The prevalence of chronic diseases such as cancer, diabetes, and bowel diseases that require ongoing medication can also drive the market growth.
Get actionable strategies to beat competition: Download Free Sample
Advancements in Minimally Invasive Procedures
Continuous innovations and developments in the field of minimally invasive surgeries have expanded the applications of various medical implants in the recent years. Procedures like laparoscopic surgeries, catheter-based therapies and robotic-assisted techniques allow for smaller incisions and faster recovery times as compared to open surgeries. This has increased acceptance for many implants that were previously only used in highly invasive procedures. For example, valves for cardiovascular diseases can be inserted through catheters instead of open-heart surgeries. Shoulder, hip and knee replacements are also increasingly performed through mini-incision or arthroscopic techniques. Implantable devices for diabetes management have also advanced with minimally invasive technology. As medical technology progresses further, more complex procedures will become feasible through minimal access points. This transformational shift towards minimally invasive approaches boosts demand for safely packaged implant components compatible with advanced tools and techniques. Growing preference for menos morbidity and patient convenience of minimally invasive surgeries can drive the medical implant market growth.
Key Takeaways from Analyst:
Global medical implant sterile packaging market growth is driven by rising geriatric population and increasing volume of surgical procedures performed worldwide. Rising number of sport and trauma injuries along with growing prevalence of chronic diseases like cardiovascular diseases and musculoskeletal disorders that require implantation of medical devices can also drive the market growth. Adoption of minimally invasive surgeries also boosts demand for sterile packaged implants and instruments.
Stringent regulatory frameworks and quality standards set by healthcare agencies can hamper the market growth. Maintaining sterility throughout the packaging and supply chain poses challenges as well as increases operational costs for manufacturers. The market also faces competition from reprocessed implants, which are cost-effective.
Developing aseptic packaging technologies that enhance ease of use and provide long term shelf life can offer opportunities for the market growth. Emerging economies of Asia Pacific and Latin America will dominate the growth owing to their large untapped population base and improving access to healthcare. China, India and Brazil are likely to experience highest demand. Moreover, favorable reimbursement scenarios and rising medical tourism will boost the adoption rates across developing countries.
Market Challenges: High cost of sterile packaging
The high cost of sterile packaging can hamper the global medical implant sterile packaging market growth. Sterile packaging involves specialized materials, machinery and processes to ensure that the implants remain completely sterilized and safe for patient use. This makes sterile packaging a highly technical and expensive endeavor. The stringent sterility and quality standards mandated by regulatory authorities increases the costs. Packaging solution providers invest heavily in state-of-the-art facilities, equipment, lab testing and quality certifications to be compliant. Advanced technologies like cold plasma, electron beam and gamma irradiation are used for sterilization, which requires large capital outlay. Frequent product validation, periodic audits and quality inspections also contribute to recurring compliance costs
Market Opportunities: Adoption of minimally invasive surgeries
Increasing adoption of minimally invasive surgical procedures across the globe can offer market growth opportunity. Minimally invasive surgeries are performed through small incisions rather than the traditional open surgeries with large incisions. This minimally invasive approach enables reduced trauma, shorter hospital stays, and quicker recovery for patients. In 2021, according to a report published by the Organization for Economic Co-operation and Development (OECD), minimally invasive procedures accounted for over 50% of total surgeries conducted in countries such as the U.S., U.K., Germany, and Japan. The advantages of minimally invasive surgeries have led to their rising popularity and broader acceptance among both patients and doctors. As the number of such complex surgeries requiring implants increases, there will be huge demand for sterile packaged implants and instruments tailored for minimally invasive techniques. Ensuring sterility during minimally invasive surgeries becomes more critical due to the narrow openings involved. This translates to heightened focus on specialized containment and packaging solutions that facilitate the use of implants through small incisions without compromising sterility. Leading medical packaging manufacturers have responded to this need by developing miniaturized sterile packaging formats, customized trays, and outer wraps suitable for minimizing the instrument footprint during minimally invasive procedures.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
By Product Type - Superior Protection and Convenience:
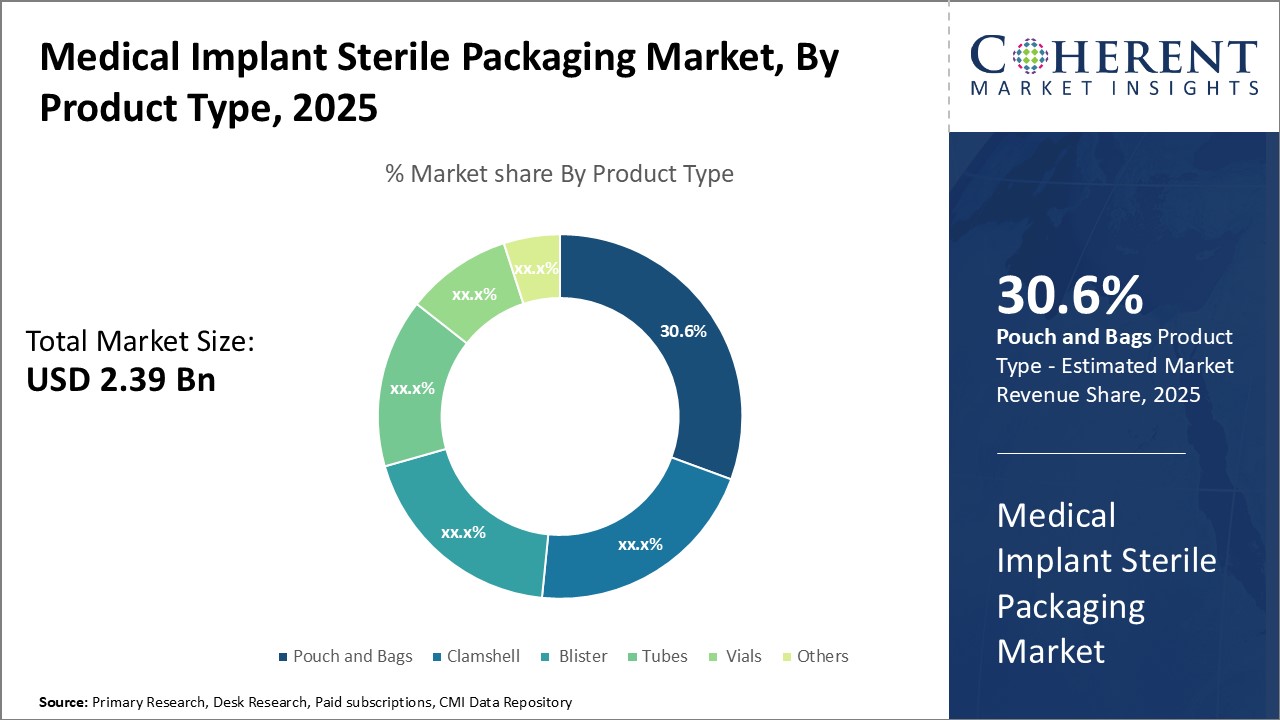
In terms of product type, pouch and bags segment is estimated to contribute the highest market share of 30.12 % in 2025, owing to their ability to securely contain implant devices while maintaining sterility and convenience. Pouches and bags are widely used due to their effectiveness in isolating implants from external contaminants during storage and transportation. Their lightweight and flexible nature allows for easy handling and organizing of multiple implants without damage. Most pouches and bags feature printed labels for clear identification and tracking of implant types. Their compact size and resealable closures provide optimal protection and permit easy opening only at the point of use during surgery. These advantages have made pouches and bags the preferred option among hospitals and healthcare facilities for packaging a variety of implant types from orthopedic screws and plates to cardiovascular stents.
By Material Type - Durability and Compatibility:
In terms of material type, plastic segment is estimated to contribute the highest market share of 40.5% in 2025, owing to its durable and compatible properties. Plastic packaging is highly resilient and withstands damage from impacts, moisture, and pressure variations during shipping and long-term shelf storage at medical facilities. A wide range of plastics such as polypropylene and polyethylene terephthalate offer effective moisture and gas barriers to avoid exposure of implants before use. Plastic also demonstrates excellent chemical resistance against cleaning and sterilization substances like ethylene oxide. Most implantable grade plastics are validated for compatibility with sterilization techniques like gamma irradiation. Their non-permeable quality assures sterility is maintained throughout distribution cycles. Plastic sealability provides reliable closure for tamper evidence and prevention of foreign particle ingress to guaranteed sterility.
By Application - Standardized Handling and Tracking:
In terms of application, spinal implants segment is estimated to contribute the highest market share of 25.71% in 2025, due to standardized packaging needs. Spinal implants packaging requires consistent sizes and formats to facilitate efficient intra-operative utilization and post- operative storage in hospital stockrooms. Uniformity in package construction, closure systems and labeling permits streamlined logistics, inventory and distribution processes at healthcare facilities. Standard pouches and trays allow for quick identification and retrieval of implant types during surgical procedures. Unique device identifiers and 2D barcodes on packaging helps track individual implants units and prevents errors. Implant packages are designed for consistent outer protection during radiation sterilization, ensuring quality remains uncompromised throughout the supply chain. Global usage of pre-determined packaging sizes and designs provides traceability and reliability in distribution of spinal implants worldwide.
Need a Different Region or Segment? Download Free Sample
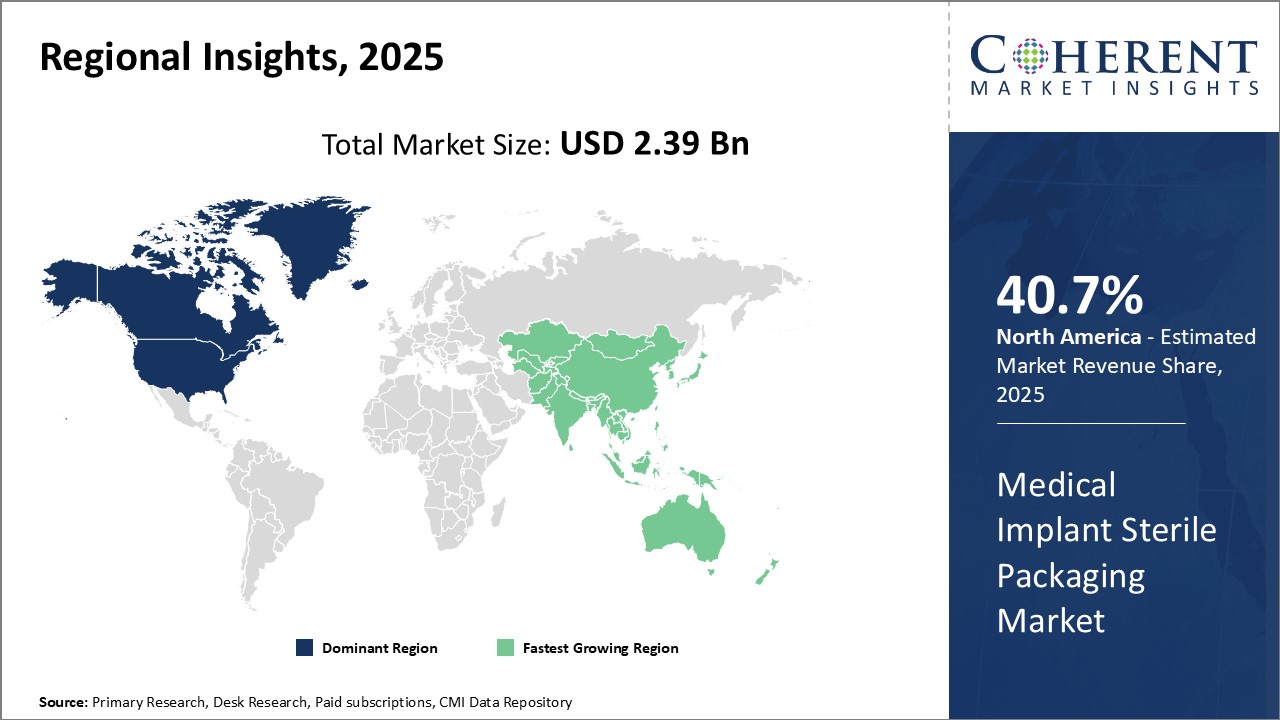
North America dominates the global medical implant sterile packaging market with an estimated market share of 40.7% in 2025. The region is home to many top medical device companies who have presence across the globe. Being an early adopter of new technologies and a presence of highly developed healthcare infrastructure, the U.S. and Canada have witnessed increased demand for various implantable medical devices. This demand has fueled the requirement for advanced sterile packaging solutions that can help maintain the sterility and integrity of packaged devices for long durations and during complex supply chain movements.
Asia Pacific region is poised to be the fastest growing market for medical implant sterile packaging. Countries like China, India, Japan and South Korea are witnessing boom in their healthcare sectors due to growing medical tourism, rising healthcare investments and expanding medical device industries. This has increased the focus on quality and sterile packaging standards to be maintained for locally manufactured as well as imported implantable devices. Rapid infrastructure development, growing affluence, aging population and improving access to healthcare can drive the market growth. The region also hosts a significant talent pool of professionals which medical device companies are leveraging to set up local manufacturing and packaging facilities.
Medical Implant Sterile Packaging Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2.39 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.3% | 2032 Value Projection: | USD 4.19 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
3M Company, Amcor Limited, Berry Global, Inc., Oliver Healthcare Packaging, Steripack Ltd., BillerudKorsnäs AB, Wipak Group, West Pharmaceutical Services, Inc., Riverside Medical Packaging Company Ltd, Renolit SE, DuPont de Nemours, Inc., Orchid Orthopedic Solutions LLC, Nelipak Healthcare Packaging, Janco Inc., Sealed Air Corporation, Sonoco Products Company, Technipaq Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: Global Medical Implant Sterile Packaging Market involves packaging products and solutions that maintain sterility and prevent contamination of medical implants and devices during storage, transportation and procedures. This market provides sterile containers, pouches, blister packs, clam shells, vials and other packaging types made from various materials like plastic, polymer and non-woven fabrics to package implants and instruments and keep them sterile until use in hospitals and surgery centers around the world.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients