Global medical device vigilance market is estimated to be valued at USD 65.65 Bn in 2025 and is expected to reach USD 133.83 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 10.7% from 2025 to 2032. Strict regulations regarding medical device safety along with the increasing usage of electronic health records can drive the market growth.
Discover market dynamics shaping the industry: Download Free Sample
Global medical device vigilance market growth is driven by growing need for companies to comply with international regulations and standards set by regulatory bodies like the U.S. FDA and the EU's MDR. Rising awareness among end users about reporting device issues and malfunctions also contributes to increase in surveillance programs being adopted across the healthcare industry. Demand for quality care by aging population and increasing expenditure on healthcare worldwide can also drive the market growth.
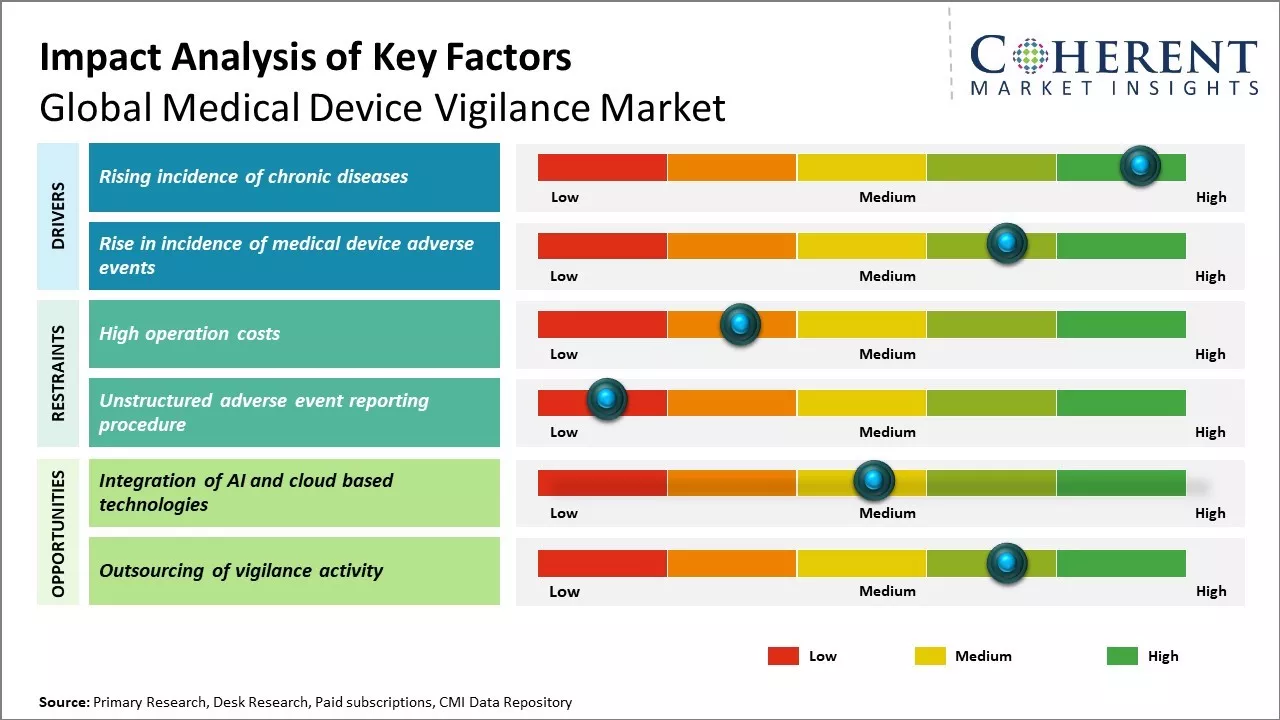
Rising incidence of chronic diseases
Chronic diseases like cancer, diabetes, cardiovascular diseases, and other poses a serious health threat. There has been increase in risk factors associated with these chronic diseases like sedentary lifestyle, smoking, obesity, stress, and others. This has led to growing incidence rates of chronic conditions. As per some estimates, chronic diseases now account for over 60% of all deaths globally. Managing these chronic illnesses requires prolonged use of medical devices like insulin pumps, pacemakers, implantable defibrillators, and others. This long term reliance on medical devices increases the chances of adverse events or device malfunctions. Device manufacturers are compelled to have robust vigilance systems to quickly detect, analyze and take corrective actions for any issues. Proactive post market surveillance allows companies to ensure patient safety and also protect their brand name.
Get actionable strategies to beat competition: Download Free Sample
Rise in incidence of medical device adverse events
Increase in occurrence of adverse events involving medical devices across healthcare facilities compels regulatory bodies to strengthen medical device vigilance practices globally. As per the data from World Health Organization, every year over 8 lakh people face serious issues including injuries or death due to faults in medical equipment. This is a matter of grave concern as devices are extensively used right from diagnosis to post-operative care. Stringent norms are being introduced to ensure continuous monitoring of performances of all devices. This has significantly boosted the need to establish robust surveillance systems for timely reporting, analysis and prevention of adverse incidents. Medical device manufacturers and healthcare providers are investing heavily in specialized vigilance software, trained workforce and setting up centralized databases. The complexity and variety of devices used also demand advanced IT tools that can efficiently process huge volumes of data. According to United Nations Population Fund, over 25% of hospitals in developing nations still lack basic equipment to deal with accidents and detect flaws in critical care appliances. Building a full-proof vigilance infrastructure has become imperative to safeguard the lives of burgeoning patient population across the world. It is predicted that in the next five years, developing economies will spend over 50 billion dollars in upgrading their vigilance standards and matching international benchmarks.
Key Takeaways from Analyst
Global medical device vigilance market growth is driven by stringent regulations for medical device safety across various regions. Stringent regulations like EU MDR and IMDRF require manufacturers to have robust vigilance systems. This will boost demand for newer vigilance software and services that help streamline case processing and reporting. North America will continue to dominate the market, owing to presence of major players and stringent regulatory environment. However, Asia Pacific region is expected to witness fastest growth due to increasing healthcare spending and expansion of medical device companies in countries like China, India and Japan.
Lack of skilled vigilance professionals can hamper the market growth. Medical device vigilance requires expertise in regulatory compliance along with clinical domain knowledge. Shortage of such talent can increase the cost of vigilance operations. Lack of harmonization between global regulations also poses challenges for companies having global operations. However, market players are focusing on providing end-to-end vigilance solutions and leveraging technologies like AI and machine learning to streamline operations. This can lower the cost of vigilance and boost adoption. Increasing mergers and acquisitions of vigilance solution providers by larger companies is a key trend in the market.
Market Challenges: High operation costs
The high operation costs associated with medical device vigilance can hamper the global medical device vigilance market growth. Vigilance of medical devices requires robust compliance with regulations and mandatory reporting of adverse incidents related to devices. This involves a comprehensive process of collecting and analyzing data from multiple sources to identify potential safety issues. Maintaining such a stringent vigilance system entails significant financial resources. Vigilance operations include setting up an entire IT infrastructure for collecting reports from various stakeholders including hospitals, physicians, and patients. This IT system needs to be constantly monitored and updated according to evolving regulatory guidelines. Staff needs to be trained extensively to handle the reporting, investigation and reporting of adverse incidents. Large workforce of medical experts is also required to clinically review each case and determine if the adverse event is indeed linked to deficiencies in device design or manufacturing. Such rigorous oversight requires round-the-clock monitoring and management of the vigilance system. All these activities associated with medical device vigilance such as IT infrastructure maintenance, staff training and clinician engagement have considerable price tags attached to them. The ongoing expenditure on operations and personnel erodes gross margins that companies generate from device sales. This deters multinational corporations from making extra investments in small market regions. According to the data published by WHO in 2021, 54 low and lower-middle income countries accounted for only 9.2% share of the global medical device market due to high costs overriding revenue prospects.
Market Opportunities: Integration of AI and cloud based technologies
The integration of artificial intelligence and cloud-based technologies provides immense opportunities for global medical device vigilance market growth. With AI and cloud, medical device manufacturers can continuously monitor patient data across distributed locations in real-time. This allows for more efficient adverse event detection and quicker response. AI algorithms can be trained on massive volumes of historical patient data compiled on the cloud. These can then recognize patterns indicating potential issues and alert clinicians. This level of ongoing remote surveillance will improve the post-market vigilance of medical devices. Cloud infrastructure also makes it easier for manufacturers and regulators to access consolidated data sources. Any concerning signals can be investigated from a centralized interface instead of retrieving paper records from multiple file rooms. Data analytics tools on the cloud can generate insights into how specific device models are faring with diverse patient populations over time. Manufacturers may identify design elements or deployment settings that need optimizing based on such real-world evidence gathered through AI and cloud. Regulatory bodies too can leverage linked data on the cloud to monitor the performance of high-risk devices more attentively. According to the report on medical device safety by World Health Organization in 2021, adopting digital health solutions is critical for improving post-market surveillance systems around the world. The cloud offers a unified platform to evaluate vigilant processes, coordinate international recalls if needed, and aid knowledge-sharing between regulatory networks. When combined with automation via AI, it can help overcome resource and infrastructure limitations that previously hindered robust vigilance.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
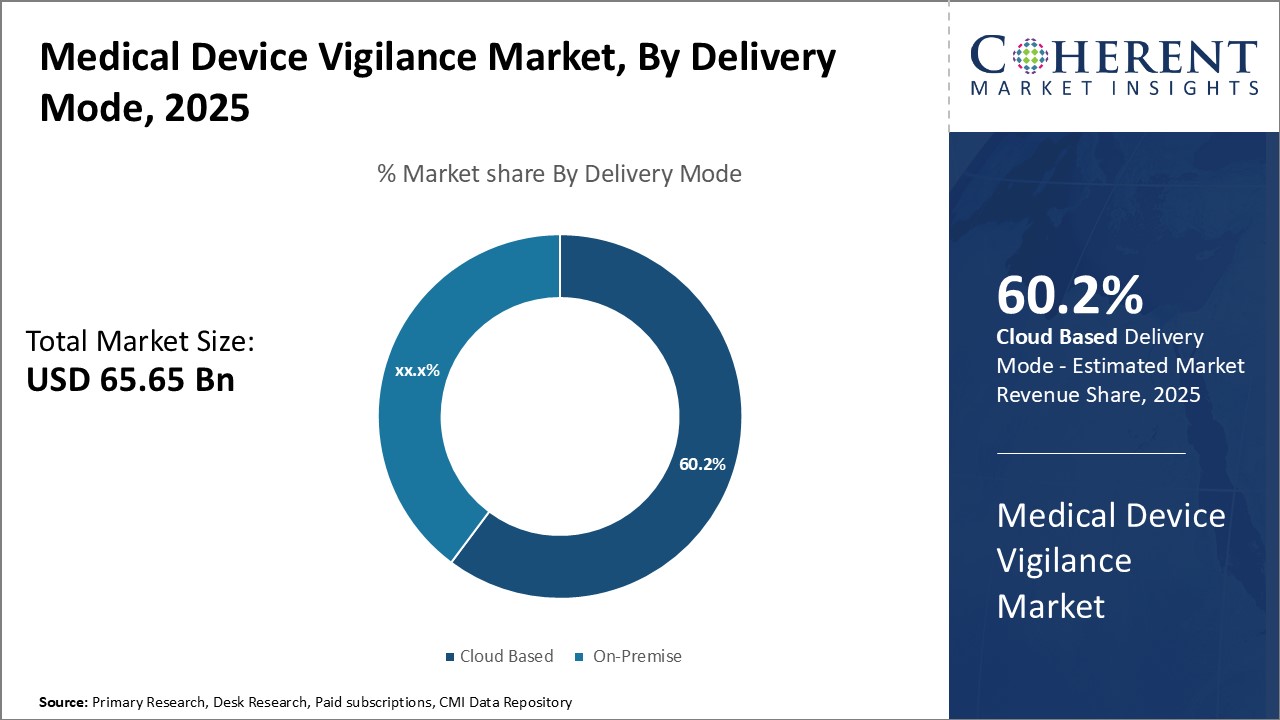
By Delivery Mode- Advancements in Cloud Technology Drives Cloud Based Segment Growth
In terms of delivery mode, cloud based segment is estimated to contribute the highest market share of 60.2% in 2025, owing to several advantages offered by cloud-based medical device vigilance solutions. Cloud deployment allows remote access to the vigilance software from any internet-enabled device, enabling round-the-clock vigilance operations. This ensures timely case intake and processing without location constraints. Furthermore, cloud solutions are cheaper to set up and maintain compared to on-premise systems as these do not require large upfront capital investments on hardware. Cloud providers also seamlessly manage software upgrades and infrastructure maintenance. The pay-as-you-go pricing of cloud services also helps vigilance teams better plan their budgets without unpredictable support and maintenance costs.
By Application- Greater Diagnostic Needs Can Drive Diagnostics Segment Growth
In terms of application, diagnostics segment is estimated to contribute the highest market share of 35.5% in 2025, owing to rise in various lifestyle diseases and growing global geriatric population. Increased prevalence of chronic illnesses such as cardiovascular diseases and diabetes has boosted demand for different diagnostic procedures worldwide. Rising healthcare awareness has also led to increased medical checkups and early disease detection efforts. This has significantly raised the volume of diagnostic tests being performed annually using medical devices. Ensuring the safety of medical diagnostic devices remains a top priority for regulatory bodies and device manufacturers to preserve public health.
By End User - Statutory Obligations Drive Original Equipment Manufacturers Segment
In terms of end user, original equipment manufacturers (OEM) segment is estimated to contribute the highest market share of 40.5% in 2025, due to strict regulatory obligations. Medical device regulations mandate OEMs to institute robust vigilance systems to monitor complaints and adverse events related to their devices. OEMs are solely responsible for timely and accurate reporting of device deficiencies and corrective actions to regulatory authorities. Non-compliance can lead to product recalls, financial penalties and even business bans. To ensure effective vigilance management from product development to post-market surveillance, most OEMs opt to outsource this function to specialized vigilance service providers. This helps them focus on core manufacturing and R&D activities while meeting all regulatory surveillance requirements.
Need a Different Region or Segment? Download Free Sample
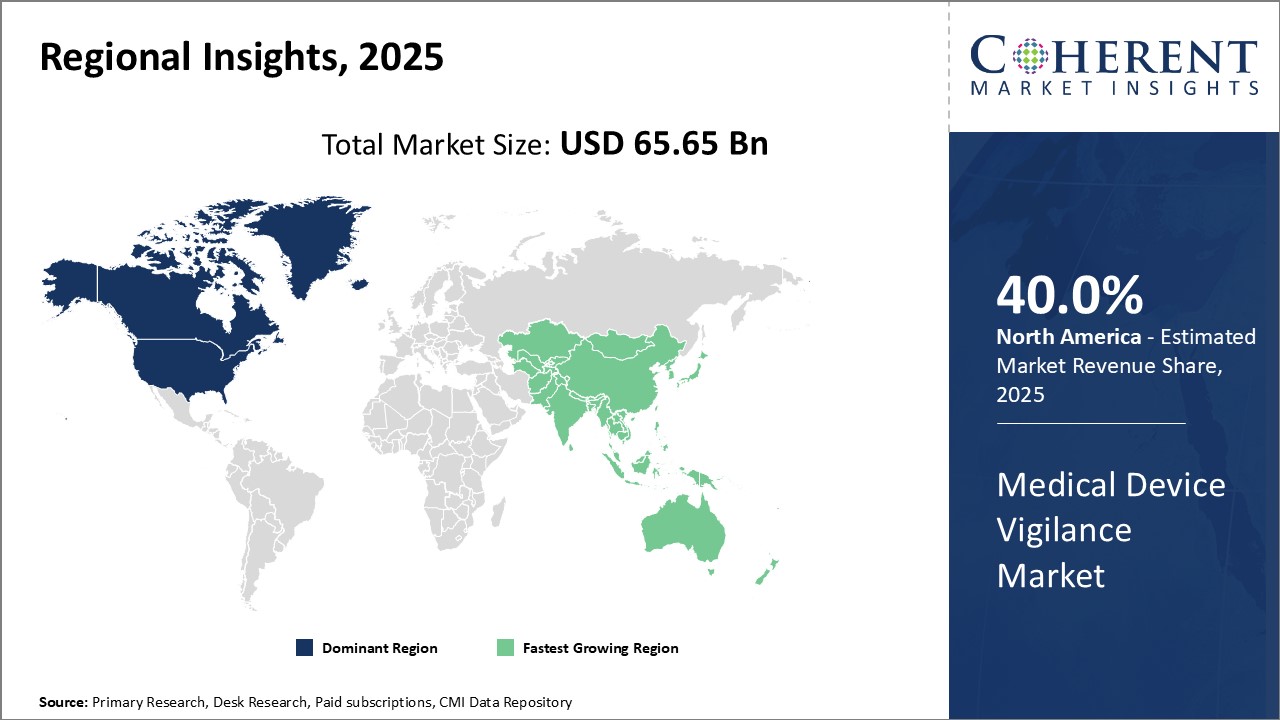
North America dominates the global medical device vigilance market with an estimated market share of 40.0% in 2025. The region enjoys the presence of many top medical device manufacturers who have their global headquarters located in the U.S. and Canada. Being an early adopter of digital health technologies, most large hospitals and healthcare systems in North America have well-established vigilance processes and reporting systems.. The region also has a highly developed pharmaceutical and medical devices industry that promotes high standards of vigilance to be followed. With significant healthcare expenditure and focus on patient safety, regulatory requirements in the U.S. and Canada ensure high levels of post-market surveillance for any adverse incidents with medical devices.
Asia Pacific region has emerged as the fastest growing market for medical device vigilance. Countries like China, Japan, India and South Korea are witnessing rising investments towards improving healthcare infrastructure and digital transformation of their health systems. This has positively impacted the growth of their vigilance capabilities. Presence of a huge patient population and rising disease incidence have boosted the need for better vigilance of medical technologies. Many global medical device majors have also established manufacturing units in the Asia Pacific to cater to the growing demand, which has facilitated improved reporting of adverse events. The region is also home to a burgeoning local medical devices industry, with companies from China, Japan and India increasingly exporting their products globally as well. This help to build their competencies as well as regulatory requirements around post-market surveillance and vigilance. With healthcare undergoing significant change across Asia Pacific, investments in this area are expected to have strong momentum.
Medical Device Vigilance Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 65.65 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.7% | 2032 Value Projection: | USD 133.83 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
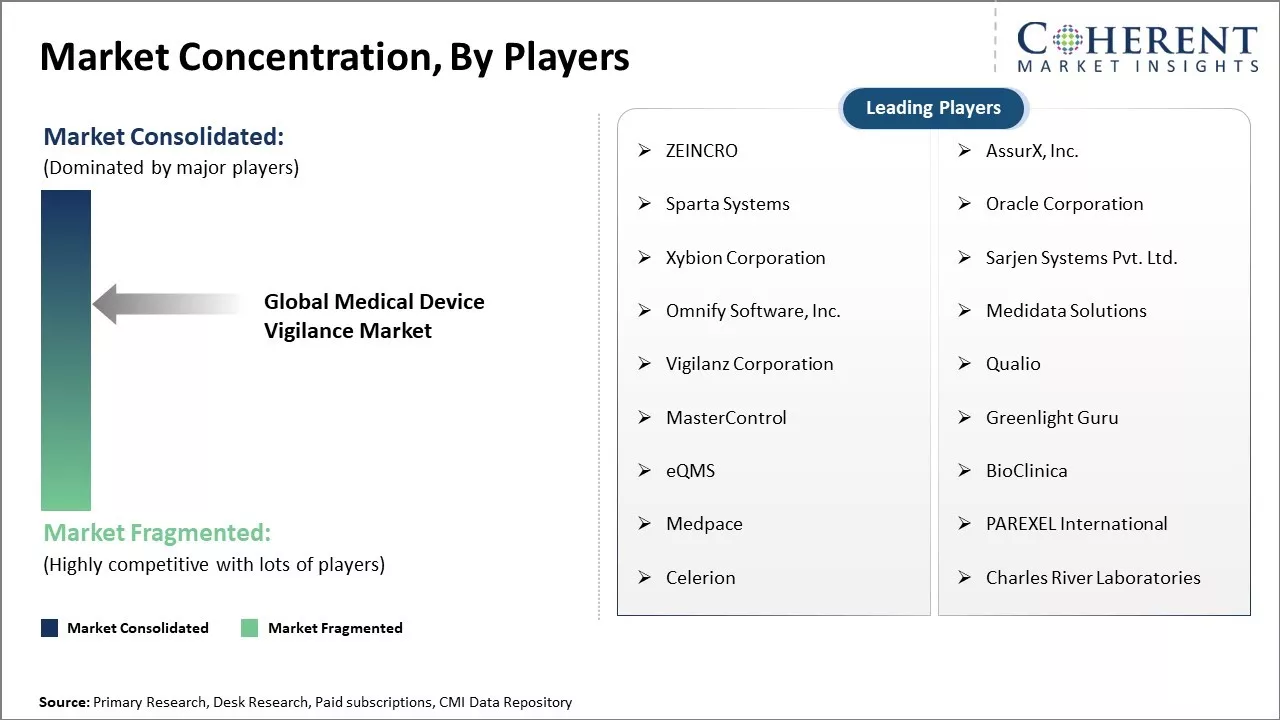
| Companies covered: |
ZEINCRO, AssurX, Inc., Sparta Systems, Oracle Corporation, Xybion Corporation, Sarjen Systems Pvt. Ltd., Omnify Software, Inc., Medidata Solutions, Vigilanz Corporation, Qualio, MasterControl, Greenlight Guru, eQMS, BioClinica, Medpace, PAREXEL International, Celerion, Charles River Laboratories |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients