Major Depressive Disorder Treatment Market Size and Forecast – 2025 – 2032
The Global Major Depressive Disorder Treatment Market size is estimated to be valued at USD 18.7 billion in 2025 and is expected to reach USD 33.3 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 8.3% from 2025 to 2032. This impressive industry size and forecast emphasize robust market growth, driven by rising prevalence rates and enhanced diagnostic capabilities globally, with North America currently holding a significant industry share.
Global Major Depressive Disorder Treatment Market Overview
Major Depressive Disorder (MDD) Treatment involves medications such as SSRIs, SNRIs, tricyclic antidepressants, MAOIs, and newer fast-acting options like esketamine nasal sprays. Non-drug therapies include transcranial magnetic stimulation (TMS), electroconvulsive therapy (ECT), and digital therapeutics that support psychotherapy. These options help manage mood, anxiety, and cognitive symptoms associated with MDD.
Key Takeaways
In the Selective Serotonin Reuptake Inhibitors segment, commanding 46.5% market share, robust research investments continue to enhance drug efficacy and safety profiles, driving sustained demand.
Oral delivery remains dominant with over 70% share, but intranasal formulations are rapidly expanding due to their rapid onset benefits. Adult patients represent the largest demographic segment, though adolescent segments are witnessing faster growth due to enhanced mental health screening programs.
Regionally, North America controls a substantial market share of approximately 38%, fueled by advanced healthcare infrastructure and high adoption of innovative therapies.
Asia Pacific is the fastest-growing region, propelled by increasing government initiatives to improve mental healthcare access and rising awareness. Europe maintains steady growth supported by favorable reimbursement and research funding in neuropsychiatric disorders.
Major Depressive Disorder Treatment Market Segmentation Analysis
To learn more about this report, Download Free Sample
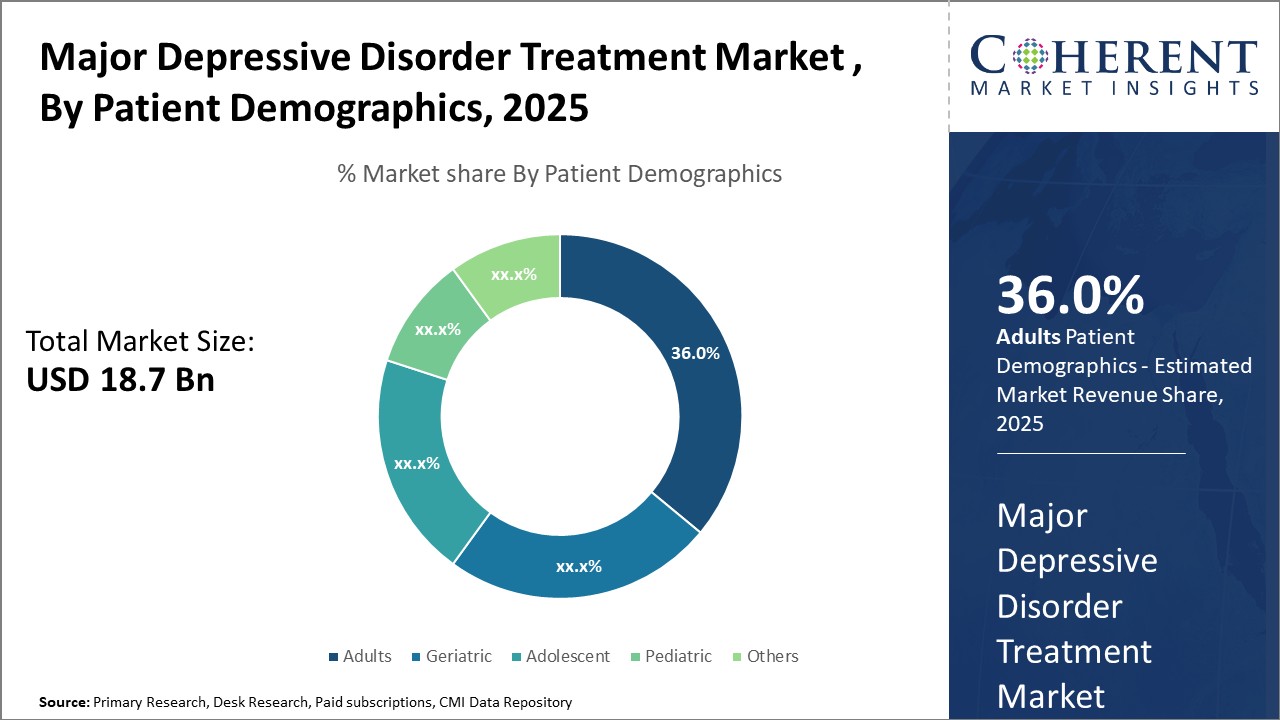
Major Depressive Disorder Treatment Market Insights, By Patient Demographics
The Patient Demographics segment encompasses Adult, Geriatric, Adolescent, Pediatric, and Others, with adults constituting the largest share at 36%, driven by the rising diagnosed cases and longevity of treatment. The adolescent segment exhibits the fastest growth rate due to increased mental health screening programs in schools and early intervention initiatives. Geriatric patients maintain steady growth with specialized treatment modifications addressing comorbidities.
Major Depressive Disorder Treatment Market Insights, By Treatment Type
In terms of Treatment Type, the SSRIs dominate the market share with 46.5%. SSRIs remain the preferred prescription due to their favorable safety profile and robust efficacy demonstrated by leading drugs such as fluoxetine and sertraline. SSRIs' expanding patient base is supported by ongoing innovations improving tolerability and minimizing withdrawal symptoms. The fastest growing subsegment is SNRIs, which are gaining traction thanks to newer compounds showing enhanced efficacy for treatment-resistant patients.
Major Depressive Disorder Treatment Market Insights, By Distribution Channel
Pharmacies dominate the market share, primarily due to patients receiving inpatient or outpatient therapeutic regimens under medical supervision. Retail Pharmacies hold a substantial share by virtue of accessibility and prescription fulfillment. Online Pharmacies constitute the fastest growing subsegment, accelerated by the surge in e-commerce and telehealth consultations, facilitating medication delivery, especially post-pandemic. Others encompass emerging delivery models, including community health programs and mobile health units targeting rural areas.
Major Depressive Disorder Treatment Market Trends
The Major Depressive Disorder Treatment market is witnessing rapid technological integration, notably through AI-powered diagnostic tools that support precision medicine approaches.
This trend was exemplified by FDA clearance of diagnostic algorithms in 2024 capable of stratifying depression severity, facilitating personalized therapy adjustments.
Additionally, investment in digital health platforms delivering virtual support for depression management increased by 35% in 2023, highlighting an industry-wide shift towards holistic care.
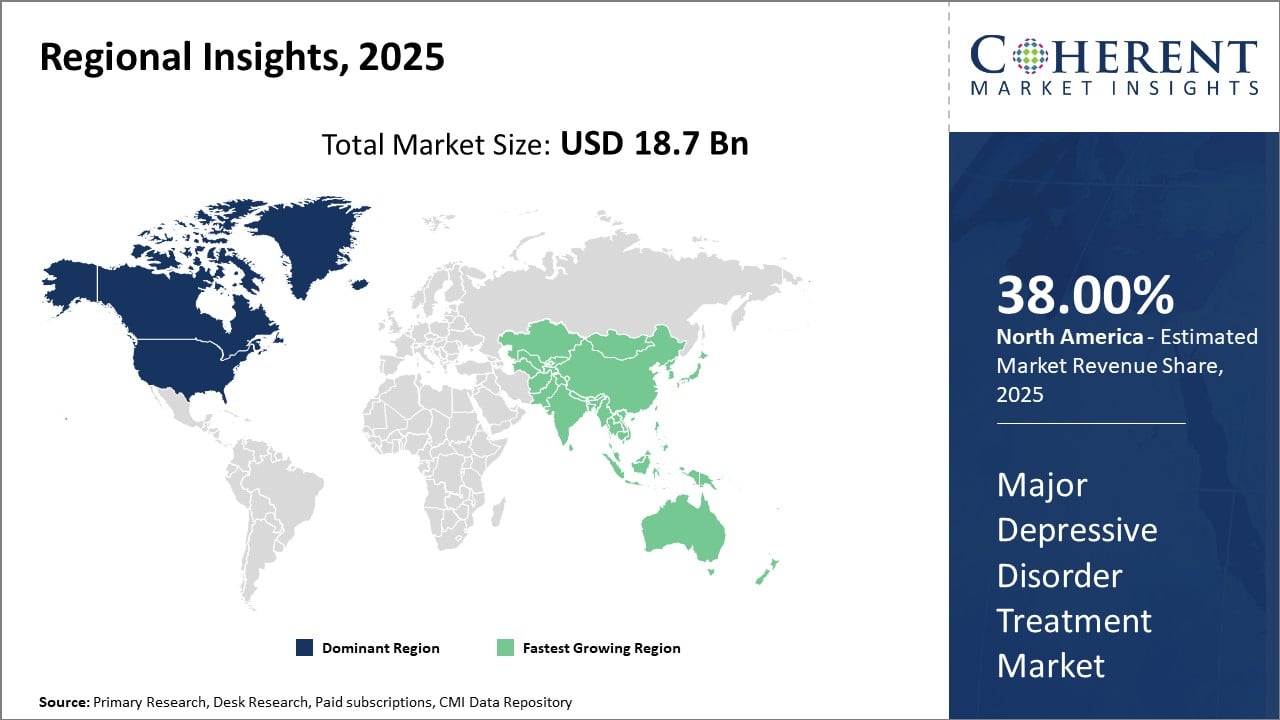
Major Depressive Disorder Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Major Depressive Disorder Treatment Market Analysis and Trends
Regionally, North America dominates the Major Depressive Disorder Treatment market, holding approximately 38% of the global market share. This dominance is supported by favorable government reimbursements, a well-established healthcare ecosystem, and leading companies headquartered in the region, such as Johnson & Johnson and Eli Lilly.
Asia Pacific Major Depressive Disorder Treatment Market Analysis and Trends
The Asia Pacific region exhibits the fastest CAGR at over 10%, driven by expanding healthcare infrastructure, increasing mental health awareness, and emerging markets like India and China implementing national mental health programs. Local pharmaceutical manufacturers are also entering strategic collaborations to penetrate this fast-growing region.
Major Depressive Disorder Treatment Market Outlook for Key Countries
USA Major Depressive Disorder Treatment Market Analysis and Trends
The USA remains the largest contributor to market revenue, owing to advanced clinical trial infrastructure and a high adoption rate of novel therapeutics such as esketamine and digital interventions. The government’s enactment of mental health parity laws and favorable insurance reimbursements have significantly expanded patient access, with over 30% of diagnosed patients receiving advanced therapies in 2024. Key market players like Pfizer and Lundbeck have reinforced their presence with pipeline expansions, supporting sustained growth.
India Major Depressive Disorder Treatment Market Analysis and Trends
India’s Major Depressive Disorder Treatment market is rapidly expanding due to a rising mental health burden and enhanced government focus on psychiatric care. Public health campaigns launched in 2024 increased diagnosis rates by 25%, propelling demand for affordable generics and novel treatment options. Local companies and multinational collaborations have introduced cost-effective oral antidepressants tailored to regional needs, positioning India as a high-growth market with evolving market dynamics.
Analyst Opinion
Supply-side indicators highlight that the expansion of production capacity in biopharmaceutical facilities is a key contributor to the increasing market revenue. For example, several companies announced capacity ramp-ups in 2024, enhancing their ability to meet demand surges for advanced antidepressants. This has positively influenced market share dynamics globally.
Demand-side analysis reveals a shift in patient preferences towards novel therapeutics such as rapid-acting antidepressants. Clinical adoption of drugs like esketamine has increased by over 40% in leading healthcare institutions during 2024, signaling changing treatment modalities and fueling market growth.
Micro-indicators suggest a surge in telepsychiatry platforms facilitating remote management of depressive disorders. Utilization rates in telehealth services have doubled since 2023 in countries like the U.S., supporting expansive market revenue streams and improved patient outreach.
Nano-level indicators identify rising research investments into personalized medicine approaches targeting genetic markers associated with treatment-resistant depression. Increased funding in this area by leading pharmaceutical companies in 2025 is expected to catalyze new market segments.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 18.7 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.3% | 2032 Value Projection: | USD 33.3 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Johnson & Johnson, Eli Lilly and Company, Pfizer Inc., AbbVie Inc., Bristol-Myers Squibb, Allergan Plc, Otsuka Pharmaceutical Co., Ltd., Lundbeck A/S, Sumitomo Dainippon Pharma Co., Ltd., Takeda Pharmaceutical Company, Sunovion Pharmaceuticals Inc., Alkermes plc | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Major Depressive Disorder Treatment Market Growth Factors
The increasing prevalence of depression worldwide continues to be a primary driver, with WHO reporting a 5.2% rise in diagnosed major depressive disorder in 2024 alone. Innovations in treatment modalities, notably the rapid adoption of ketamine-based therapies, have accelerated clinical uptake due to their efficacy in treatment-resistant cases. Additionally, expanding reimbursement policies in North America and Europe have improved patient access, facilitating greater market penetration. The emergence of AI-enabled diagnostic tools is also enhancing early detection rates, further propelling market revenue growth.
Major Depressive Disorder Treatment Market Development
In January 2025, the U.S. FDA expanded approval of SPRAVATO (esketamine nasal spray) so it can now be used as a standalone treatment (monotherapy) for adults with treatment-resistant depression, eliminating the prior requirement to use it with an oral antidepressant.
In September 2025, NeuroKaire, a biotechnology company, introduced BrightKaire—a test utilizing “brain in a dish” technology designed to assist clinicians in selecting the most effective antidepressant treatment for individuals with major depressive disorder (MDD).
Key Players
Leading Companies of the Market
Johnson & Johnson
Eli Lilly and Company
Pfizer Inc.
Bristol-Myers Squibb
Allergan Plc
Otsuka Pharmaceutical Co., Ltd.
Lundbeck A/S
Sumitomo Dainippon Pharma Co., Ltd.
Takeda Pharmaceutical Company
Sunovion Pharmaceuticals Inc.
Alkermes plc
Several leading companies have strategically invested in fast-track clinical trials and expanded their product pipelines with next-generation therapeutics. For instance, Eli Lilly’s 2024 launch of a novel oral antidepressant demonstrated a 15% improvement in remission rates, boosting its market share. Meanwhile, Johnson & Johnson’s collaboration with digital therapeutics platforms has enhanced patient adherence, resulting in notable business growth.
Major Depressive Disorder Treatment Market Future Outlook
The future of the Major Depressive Disorder treatment market is expected to be shaped by innovation in rapid-acting agents, psychedelic-assisted therapies, and advanced neuromodulation. Psychedelic-derived compounds and NMDA receptor modulators are progressing through late-stage clinical trials, holding potential to transform treatment for patients with treatment-resistant depression. Digital therapeutics and AI-driven care platforms are also emerging as complementary options to traditional therapies. As pharmaceutical companies pursue licensing and M&A in the mental health space, competition is likely to intensify, leading to broader patient access and more personalized treatment pathways. Overall, the market is set to expand beyond conventional drugs toward a more integrated and diversified treatment ecosystem.
Major Depressive Disorder Treatment Market Historical Analysis
Traditionally, the MDD treatment landscape has been dominated by pharmacological therapies such as SSRIs, SNRIs, and atypical antidepressants, supported by psychotherapy and electroconvulsive therapy. While these drugs improved tolerability over time, high rates of partial response and treatment resistance highlighted persistent unmet needs. The past decade witnessed gradual diversification with the introduction of neuromodulation therapies such as repetitive transcranial magnetic stimulation (rTMS), as well as the approval of esketamine, which represented a major milestone in rapid-acting antidepressants. Academic and clinical research consistently pointed toward the limitations of monoaminergic drugs, prompting investment into novel mechanisms.
Sources
Databases:
World Health Organization (WHO) Mental Health Database
National Institute of Mental Health (NIMH) Statistics
GlobalData CNS Therapy Reports
Magazines:
Psychiatric Times
Psychology Today
Medical News Today (Psychiatry Section)
PharmaTimes
Journals:
Journal of Affective Disorders (Elsevier)
The American Journal of Psychiatry (APA Publishing)
JAMA Psychiatry
Neuropsychopharmacology (Nature Publishing)
Newspapers:
The Guardian (Health & Science)
The Washington Post (Health Section)
The Economic Times (Pharma & Healthcare)
The New York Times (Mental Health Coverage)
Associations:
American Psychiatric Association (APA)
World Psychiatric Association (WPA)
European College of Neuropsychopharmacology (ECNP)
National Alliance on Mental Illness (NAMI)
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients