Magnetic Driven Drug Delivery Technology Market Size and Forecast – 2026 – 2033
The Global Magnetic Driven Drug Delivery Technology Market size is estimated to be valued at USD 3.5 billion in 2026 and is expected to reach USD 7.8 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 11.8% from 2026 to 2023.
Global Magnetic Driven Drug Delivery Technology Market Overview
Magnetic-driven drug delivery technology products use magnetic fields to guide and control the movement of drug-loaded carriers within the body. These systems typically consist of magnetic nanoparticles or micro-carriers combined with external magnetic devices that direct drugs to targeted tissues or organs. The technology is designed to improve drug localization, reduce systemic side effects, and enhance therapeutic efficiency. It is applied in oncology, localized infections, and targeted therapies, often integrated with imaging systems for precise positioning and controlled drug release.
Key Takeaways
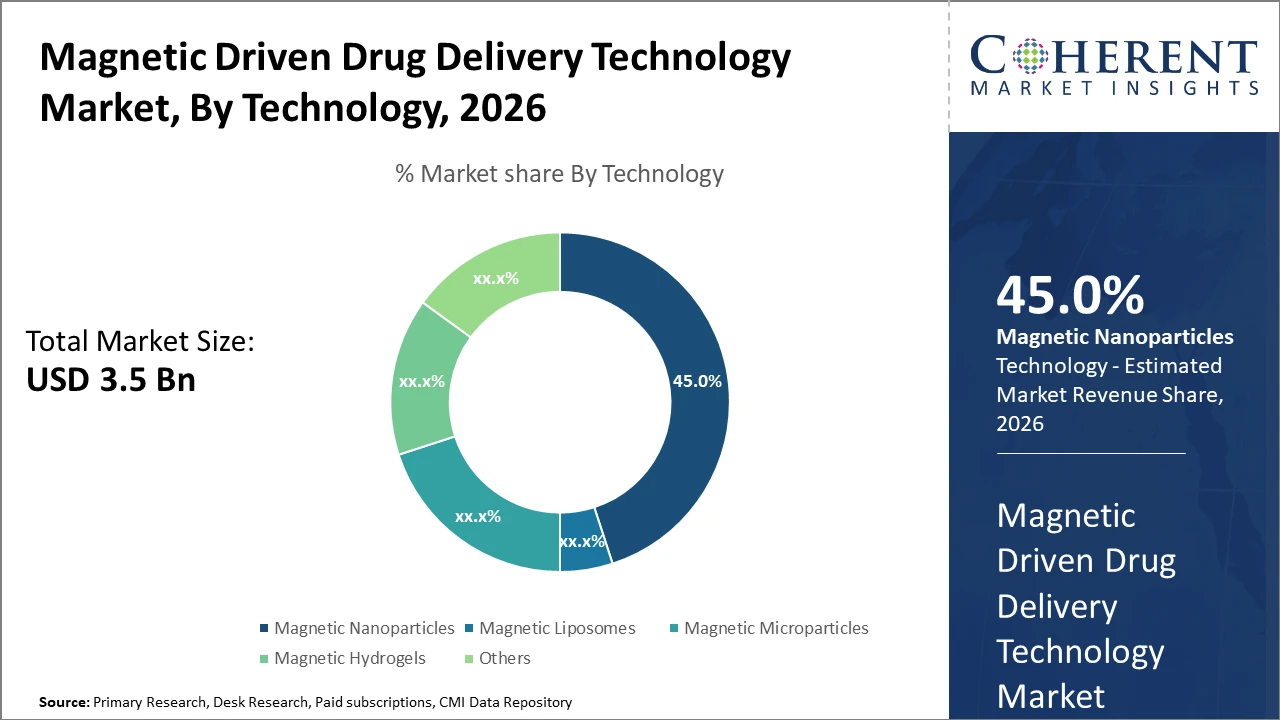
The magnetic nanoparticles segment holds a dominant market share at 45%, driven by superior target specificity and ease of functionalization.
Oncology applications continue to lead segment growth owing to rising cancer incidence and robust clinical validation of magnetic delivery platforms.
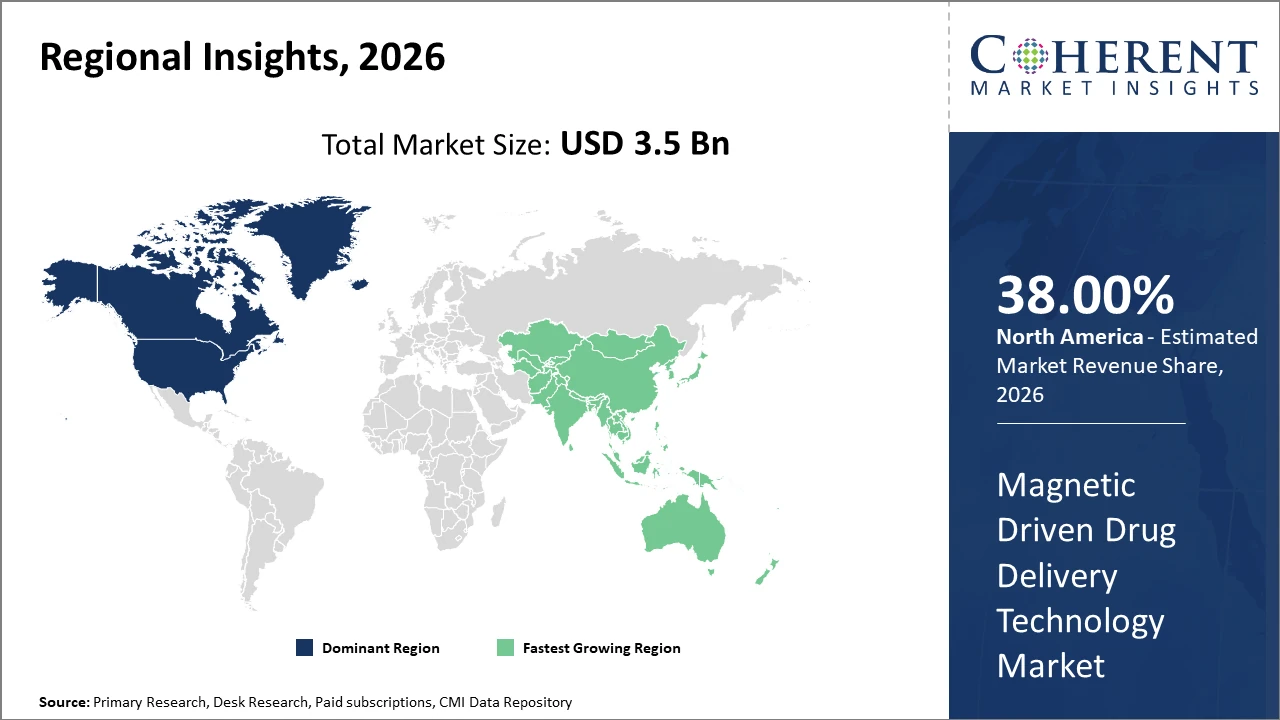
North America retains its dominance due to advanced healthcare infrastructure and accelerated regulatory pathways, accounting for approximately 38% of the market share.
Asia Pacific exhibits the fastest CAGR driven by expanding healthcare investments, increasing disease burden, and local manufacturing scale-ups, notably in China and India.
Magnetic Driven Drug Delivery Technology Market Segmentation Analysis
To learn more about this report, Download Free Sample
Magnetic Driven Drug Delivery Technology Market Insights, By Technology Type
Magnetic Nanoparticles dominate the market share at 45%. Magnetic Nanoparticles have established supremacy due to their superior magnetic responsiveness, biocompatibility, and ease of functionalization, making them ideal for precise drug targeting in complex diseases. The fastest-growing subsegment, Magnetic Liposomes, is gaining ground due to its capability to encapsulate both hydrophilic and hydrophobic drugs, enhancing payload versatility and delivery control. Magnetic Microparticles and Magnetic Hydrogels have niche applications focused on localized drug release and tissue engineering, respectively, while Others include emerging technologies such as magnetic nanofibers with specialized applications.
Magnetic Driven Drug Delivery Technology Market Insights, By Application
Oncology holds the dominant share due to the high clinical demand and proven efficacy of magnetic delivery systems in tumor targeting. The fastest-growing application is Neurological Disorders, driven by advancements in crossing the blood-brain barrier using magnetic nanoparticle vectors, addressing unmet medical needs. Cardiovascular applications offer moderate growth with ongoing trials in magnetically guided thrombolytic delivery, while Infectious Diseases and Others continue to gradually gain attention through research into site-specific antimicrobial delivery and gene therapy.
Magnetic Driven Drug Delivery Technology Market Insights, By End-User
Hospitals dominate the market share, fueled by the increasing incorporation of magnetic drug delivery technologies in clinical settings for advanced therapies. The fastest-growing end-user segment is Pharmaceutical Companies investing in in-house R&D and partnerships to expand pipelines with magnetic delivery systems. Research Institutes sustain steady growth driven by innovations and clinical trials exploring new magnetic formulations.
Magnetic Driven Drug Delivery Technology Market Trends
The evolving Magnetic Driven Drug Delivery Technology market is marked by the rise of multi-functional magnetic carriers capable of simultaneous diagnosis and therapy, fostering theranostic applications as evidenced by MagForce AG’s latest product launch in 2025.
Additionally, growing adoption of biodegradable magnetic nanoparticles has set new safety benchmarks, particularly in neurological therapies, as demonstrated in recent clinical data from U.S. medical centers in 2024.
Furthermore, AI-enabled remote control of magnetic fields ensures precise delivery mechanisms, optimizing therapeutic windows and minimizing exposure risks, with pilot results showcased by Endomagnetics Ltd.
Magnetic Driven Drug Delivery Technology Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Magnetic Driven Drug Delivery Technology Market Analysis and Trends
In North America, the dominance in the Magnetic Driven Drug Delivery Technology market is underpinned by advanced healthcare infrastructure, substantial R&D investment, and favorable regulatory frameworks. The region accounts for approximately 38% of the market share, with leading companies like Siemens Healthineers and MagForce AG driving innovation through extensive clinical collaborations and pilot projects.
Asia Pacific Magnetic Driven Drug Delivery Technology Market Analysis and Trends
Meanwhile, Asia Pacific exhibits the fastest growth rate, propelled by rising disease prevalence, government incentives for nanomedicine, and expanding local manufacturing units, particularly in China and India. The CAGR in this region exceeds 13%, fueled by supportive health policies and increasing penetration of low-cost magnetic drug delivery solutions.
Magnetic Driven Drug Delivery Technology Market Outlook for Key Countries
USA Magnetic Driven Drug Delivery Technology Market Analysis and Trends
The U.S. market leads with high adoption rates of magnetic drug delivery platforms supported by robust clinical research, evidenced by over 60 active trials investigating magnetic nanoparticles in cancer treatment in 2024. Reimbursement policies favoring innovative therapeutics and collaborations between biotech firms and healthcare providers are driving market revenue growth. Major market players, including Siemens Healthineers and Endomagnetics Ltd, have ramped up production and distribution infrastructure, fostering widespread clinical acceptance and reinforcing the U.S. position as a market innovation hub.
China Magnetic Driven Drug Delivery Technology Market Analysis and Trends
China’s market has seen exponential expansion, with government policies underscoring nanomedicine innovation, supporting over 20% year-on-year revenue increase from magnetic drug delivery technologies. Local companies like NanoCarrier Co. Ltd have invested heavily in large-scale nanoparticle manufacturing facilities, enabling cost-competitive products. A surge in chronic disease incidence alongside rising healthcare access in tier-2 and tier-3 cities has created fertile grounds for market growth, positioning China as a leading force in Asia Pacific.
Analyst Opinion
Precision Enhancement and Therapeutic Index Optimization: Recent studies in 2024 indicate a 35% improvement in therapeutic efficacy when magnetic-driven drug delivery systems are applied in targeted cancer treatments, sharply reducing off-target toxicity. These results strongly contribute to the increase in market share as clinical adoption rises, particularly in oncology.
Expansion in Nanoparticle Synthesis Capacity: Manufacturing capacities of magnetically responsive nanoparticles experienced an upsurge of 28% in 2025 due to innovations in cost-effective synthesis methods and greater process automation. This production capacity growth supports a stable supply chain, directly influencing positive market revenue trends in Asia Pacific and North America.
Diverse Clinical Applications Driving Demand: Demand-side indicators reveal expanding application domains, including cardiovascular diseases and central nervous system disorders, with clinical trials registering a 22% increase in 2024 alone. This diversification underpins the sustained market growth by broadening the use cases across therapeutic specialties.
Regulatory Approvals and Reimbursement Policies: Regulatory milestones attained in 2025, including expedited reviews and approvals by leading agencies for key magnetic nanoparticle-based delivery products, have propelled market dynamics. In parallel, reimbursement frameworks favoring innovative drug delivery technologies notably amplify business growth in developed markets like the U.S. and Europe.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 3.5 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 11.8% | 2033 Value Projection: | USD 7.8 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Micromod Partikeltechnologie GmbH, Ocean NanoTech LLC, Advanced Magnetics Inc., Remanci Labs, QuantumDx Group, Persistent Systems Ltd, Magnet Solutions Inc., Ferrotec Corporation, Vaxxas Pty Ltd, BIND Therapeutics | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Magnetic Driven Drug Delivery Technology Market Growth Factors
One key growth driver is the rising prevalence of cancer and chronic diseases worldwide, which has magnified the demand for targeted drug delivery systems, accounting for over 40% of new product pipelines utilizing magnetically driven technologies. Additionally, advancements in magnetic nanoparticle fabrication, improving biocompatibility and magnetic responsivity, have accelerated clinical translation and adoption. The increasing government initiatives supporting nanomedicine innovation, particularly in North America and Asia, have provided vital funding and regulatory facilitation, enhancing market revenue streams. Furthermore, integration of artificial intelligence with magnetic drug delivery platforms for personalized treatment protocols is emerging strongly, evidenced by recent pilot projects in 2024 that achieved enhanced delivery precision.
Magnetic Driven Drug Delivery Technology Market Development
In April 2024, Ferronova initiated the MAGMAP clinical trial to evaluate its FerroTrace® magnetic nanoparticle technology in patients with gastric and esophageal cancers. The study focuses on assessing the safety and diagnostic performance of FerroTrace® for identifying high-risk lymph node regions, representing an important clinical milestone in the broader use of magnetic nanoparticles for precision cancer mapping and image-guided interventions.
In June 2023, Stevanato Group further detailed the Vertiva™ On-Body Delivery System, highlighting its reusable electronic controller paired with a single-use disposable pod. The magnetically coupled drive mechanism allows controlled, reliable infusion over extended periods, positioning Vertiva as a flexible platform for patient-centric, self-administration of injectable therapies.
Key Players
Leading Companies of the Market
Micromod Partikeltechnologie GmbH
Ocean NanoTech LLC
Advanced Magnetics Inc.
Remanci Labs
QuantumDx Group
Persistent Systems Ltd
Magnet Solutions Inc.
Ferrotec Corporation
Vaxxas Pty Ltd
BIND Therapeutics
Leading companies have embraced differentiated market growth strategies such as collaborative R&D partnerships and expansion into emerging economies. For instance, MagForce AG’s strategic alliance with U.S.-based oncology centers facilitated rapid clinical adoption, resulting in a 17% surge in their market share in 2024. Similarly, Nanocarrier’s investment in scalable nanoparticle manufacturing catalyzed their revenue growth by 25% in Asia Pacific markets during 2025, illustrating successful market penetration approaches.
Magnetic Driven Drug Delivery Technology Market Future Outlook
The future outlook is promising as magnetic drug delivery systems move closer to clinical viability for a range of applications, including oncology, localized infections, and neurological disorders. Continued innovation in magnetic nanoparticle design, bioresponsive materials, and real-time imaging integration will enhance targeting accuracy and therapy personalization. External magnetic field devices are expected to become more compact, tunable, and compatible with existing clinical imaging systems. Regulatory frameworks are likely to evolve to address safety, standardization, and quality control for these complex combination products. As precision medicine and minimally invasive therapies gain priority, magnetic driven systems are poised to become an important component of next-generation therapeutic platforms.
Magnetic Driven Drug Delivery Technology Market Historical Analysis
The magnetic driven drug delivery technology market has developed from basic research in targeted delivery mechanisms and magnetic manipulation of therapeutic agents. Early studies in magnetically guided delivery focused on external control of magnetic nanoparticles carrying drugs to specific anatomical sites, with an eye toward reducing systemic side effects and improving therapeutic concentration at disease loci. Initial research faced challenges related to particle biocompatibility, magnetic field control, and targeted release kinetics. Over time, advances in nanotechnology, polymer chemistry, and external magnetic field engineering enabled practical prototypes capable of navigating complex biological environments. Preclinical and clinical research refined strategies for tumor-targeted delivery, site-specific release, and controlled therapeutic release. Adoption was initially limited to specialized research settings due to technological complexity and regulatory scrutiny.
Sources
Primary Research Interviews:
Nanotechnology researchers
biomedical engineers
pharmaceutical R&D professionals
Databases:
NIH Nanomedicine Data
ClinicalTrials.gov
FDA Drug Delivery Systems
Statista Nanotechnology Data
ScienceDirect
Magazines:
Nanotechnology Today
Drug Delivery Technology
Pharmaceutical Technology
BioTechniques
MedTech Insight
Journals:
Journal of Controlled Release
Nanomedicine
Advanced Drug Delivery Reviews
Biomaterials
ACS Nano
Newspapers:
Financial Times (Science)
Reuters Healthcare
The Guardian (Technology)
The New York Times (Science)
Bloomberg Health
Associations:
Controlled Release Society
American Institute for Medical and Biological Engineering
International Society for Nanomedicine
Materials Research Society
Biomedical Engineering Society
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients