Lysosomal Storage Diseases Therapeutics Market is estimated to be valued at USD 11.6 Bn in 2025 and is expected to reach USD 20.4 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 8.4% from 2025 to 2032.
Analysts’ Views on Global Lysosomal Storage Diseases Therapeutics Market :
Increasing prevalence of lysosomal storage diseases, increasing products launch can drive the growth of global lysosomal storage diseases (LSD) therapeutics market. Lysosomal storage diseases are believed to have an estimated frequency of about one in every 5,000 live births. For instance, according to an article published by Molecular Genetics and Metabolism in July 2021, study was conducted to check prevalence of patients with lysosomal storage disorders and peroxisomal disorders by conducting a nationwide survey in Japan. A total of 13,304 hospitals with more than 20 hospitalized beds in Japan were surveyed for their experience in treating patients with LSD. The number of patients was estimated by calculating the rate/frequency of overlap. The estimated number of patients was 1658 for Fabry disease, 72 for mucopolysaccharidosis I, 275 for mucopolysaccharidosis II, 211 for Gaucher disease, 124 for Pompe disease, 83 for metachromatic leukodystrophy, 57 for Niemann-Pick type C, and 262 for adrenoleukodystrophy. In addition the birth prevalence was calculated using the estimated number of patients and birth year data for each disease, and was 1.25 for Fabry disease, 0.09 for mucopolysaccharidosis I, 0.38 for mucopolysaccharidosis II, 0.19 for Gaucher disease, 0.14 for Pompe disease, 0.16 for metachromatic leukodystrophy, 0.16 for Niemann-Pick type C, and 0.20 for adrenoleukodystrophy.
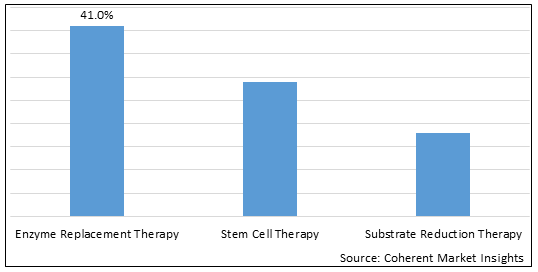
Figure 1. Global Lysosomal Storage Diseases Therapeutics Market Share (%), by Product Type, 2025
To learn more about this report, Download Free Sample
Global Lysosomal Storage Diseases Therapeutics Market– Driver
Increasing prevalence of lysosomal storage diseases
The growing incidences of lysosomal storage diseases among people are a major factor leading to the high demand for lysosomal storage diseases therapeutics. For instance, according to the report published by the National Organization for Rare Disorders on February 8, 2021, out of 4 million infants screened, over 12,000 newborns are found to have a rare disorder that, if left undiagnosed and untreated, would cause severe developmental disability or death. This report is as current as of November 2022.
Launches of newer products by key market players for lysosomal storage disease
The increasing research and development activities to invent newer therapies that can treat people with lysosomal storage disease can drive the growth of market. For instance, on February 8, 2021, the U.S. Food and Drug Administration (FDA) granted orphan drug status to two M6P gene therapies. One is intended for Gauchers disease and another for treatment of the inherited metabolic disorder mucolipidosis.
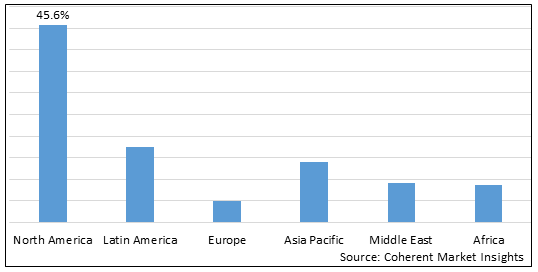
Figure 2. Global Lysosomal Storage Diseases Therapeutics Market Value (US$ Billion), by Region, 2025
To learn more about this report, Download Free Sample
Global Lysosomal Storage Diseases Therapeutics Market - Regional Analysis
Among regions, North America is estimated to hold a dominant position in the global lysosomal storage diseases therapeutics market over the forecast period. This is due to the increasing research and development activirties for treatment of lysosomal storage disease. For instance, on February 9, 2025, Regenxbio, a U.S. based biotechnology company presents additional positive interim data from phase I/II trial of RGX-121 for the treatment of Hunter Syndrome at 18th Annual WORLD Symposium 2022. The gene therapy was found to be well tolerated with no drug related serious adverse events across three dose level. Dose-dependent reductions in Cerebrospinal fluid biomarkers continued neurodevelopmental after RGX-121 administration and evidence of systemic enzyme expression and biomarker activity was observed. They are further planning expansion using commercial-scale Current good manufacturing practices (cGMP) material in 2022.
Global Lysosomal Storage Diseases Therapeutics Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others are facing problems with the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a negative impact on the global lysosomal storage diseases therapeutics market. This is because of the disruption in the treatment due to COVID related restrictions. According to an article published in molecular genetics and metabolism on April 24, 2020, impact of COVID-19 related healthcare crisis on treatments for patients with lysosomal storage disorders were studied in Italy by Regional Coordinating Center for Rare Diseases, University Hospital of Udine, Italy and Institute of Hygiene and Clinical Epidemiology, Italy. 49 % of patients receiving enzyme replacement therapy in hospitals experienced disruptions, versus 6% of those home-treated. The main reasons of missed infusions were fear of infection (62.9%) and re-organization of the infusion centers (37%).
Global Lysosomal Storage Diseases Therapeutics Market Segmentation:
The global lysosomal storage diseases therapeutics market report is segmented into treatment, Indication, End users and Region.
Based on Treatment, the global lysosomal storage diseases therapeutics market is segmented into enzyme replacement therapy, stem cell therapy, substrate reduction therapy, and others. Out of which, the enzyme replacement therapy is expected to dominate the market due to newer approvals and launches of therapies in this field.
Based on Indication, the global lysosomal storage diseases therapeutics market is segmented into gaucher's disease, fabry disease, pompe’s syndrome, mucopolysaccharidosis, others. The gaucher’s disease segment is expected to dominate the market over the forecast period and this is attributed to widely infected population by gaucher’s disease.
Based on End user, the global lysosomal storage diseases therapeutics market is segmented into hospitals, and clinics. The hospital segment is expected to dominate the market over the forecast period and this is due to the increasing numbers of hospitals for the treatment of rare diseases.
Among all segmentation, the treatment segment has the highest potential due to the increasing approval for research and development activities by the key market players. For instance, on January 5, 2023, Orchard Therapeutics PLC, a U.K. based biotechnology company, announced the U.S. Food and Drug Administration (FDA) has cleared its Investigational New Drug (IND) application for OTL-203, a hematopoietic stem cell (HSC) gene therapy being developed for the treatment of the Hurler subtype of mucopolysaccharidosis type I. The company will starts its trials to evaluate safety and efficacy of OTL-203 in second half of 2023.
Lysosomal Storage Diseases Therapeutics Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 11.6 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.4% | 2032 Value Projection: | USD 20.4 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Shire plc, Pfizer, Inc., Sanofi, BioMarin Pharmaceutical Inc., Actelion Ltd., Raptor Pharmaceutical Corp., Protalix Biotherapeutics Inc., Quest Diagnostics, and Amicus Therapeutics, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Lysosomal Storage Diseases Therapeutics Market Cross Sectional Analysis:
Increase in approval of newer therapies in Asia Pacific region is expected to drive growth of treatment segment in this region. For instance, on March 23, 2021, JCR Pharmaceuticals, a Japan-based Pharmaceutical company, announced approval of IZCARGO (pabinafusp alfa 10 mL, intravenous drip infusion) for the treatment of mucopolysaccharidosis type II (MPS II). IZCARGO is a recombinant iduronate-2-sulfatase enzyme replacement therapy (ERT) that relies on J-Brain Cargo, a proprietary technology developed by JCR, to deliver therapeutics across the blood-brain barrier (BBB). It is the first-ever approved ERT that penetrates the BBB via intravenous administration, a potentially life-changing benefit for individuals with lysosomal storage disorders (LSDs) such as MPS II.
Global Lysosomal Storage Diseases Therapeutics Market : Key Developments
On February 9, 2022, Avrobio, a U.S.-based pharmaceutical company, announced that its gene therapy for rare lysosomal storage disorder showed potential durability in the first three patients more than one year after infusion. The gene therapy is given for cystinosis which is treated with cysteamine, a common treatment that reduces the buildup of cystine crystals in the kidneys for people with the inherited disease. The first three patients on the gene therapy with AVR-RD-04, remain off oral cysteamine between 12 and 26 months after the treatment infusion. A fourth patient received the therapy in November 2021. None of the patients have experienced adverse events due to the treatment, but have had mild or moderate side affects due to stem cell mobilization, underlying disease, preexisting conditions or myeloablative condition.
On March 1, 2023, Sanofi, a France-based pharmaceutical company, on occasion of Rare Disease Day announced that it will launch new products for rare disease in India by end of 2023 or beginning of next year. The company has also received a recommendation from the Subject Expert Committee to import and market two new products in India, Nexviazyme (Avalglucosidase alfa powder) and Xenpozyme (Olipudase alfa powder) for Pompe disease and Niemann-Pick disease (ASMD) respectively.
On March 25, 2020, IntraBio Inc., U.K. based biopharmaceutical company shared that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation to its lead compound (IB1001) for the treatment of Niemann-Pick disease Type C (NPC). IB1001 (N-acetyl-L-leucine) is currently being investigated for the symptomatic, and disease-modifying, neuroprotective treatment of NPC in a multinational clinical trial. In addition to the Fast Track Designation, IB1001 has previously received Orphan Drug Designations in the U.S. (FDA) and EU (European Commission) and granted a Rare Pediatric Disease Designation in the US (FDA) for the treatment of NPC.
On May 20, 2022, European Medicine Agency recommended granting marketing authorization in European Union for Xenpozyme (olipudase alfa) a therapy for the treatment of non-central nervous system (CNS) manifestations of Acid Sphingomyelinase Deficiency (ASMD), a rare and progressive genetic disease. Xenpozyme is product of Sanofi, a Franced-based pharmaceutical company’s subsidery company : Genzyme Europe B.V., Netherlands based biotechnological company. It is indicated for use in children and adults of all ages with type A/B or type B ASMD.ASMD is also known as Niemann-Pick Disease, a rare genetic metabolic disorder or lysosomal storage disorder.
Global Lysosomal Storage Diseases Therapeutics Market : Key Trends
On March 7, 2023, an e-learning programme was launched to help healthcare professionals better identify lysosomal storage disorders (LSDs) and help provide faster diagnosis and access to treatment. Expert nurses and health care professionals from several lysosomal disorders centres across the U.K. designed the e-Learning programme with input from the British Inherited Metabolic Disease Group (BIMDG), who are hosting the training on their website. The training is open to LSD nurses and allied health professionals through membership of BIMDG and is accredited by the Royal College of Nursing (RCN). This means that participants can earn Continuing Professional Development (CPD) points by completing the modules.
On November 9, 2020, Axovant Gene Therapies, a U.S.-based clinical-stage pharmaceutical company, announced that the U.S. Food and Drug Administration has lifted its clinical hold and cleared Axovant’s Investigational New Drug (IND) application to initiate a registrational study of gene therapy to treat patients with Tay-Sachs disease and Sandhoff disease. The Axovant therapy, AXO-AAV-GM2, is the first investigational gene therapy to achieve IND clearance for Tay-Sachs and Sandhoff.
Global Lysosomal Storage Diseases Therapeutics Market : Restraint
High prices of Lysosomal Storage Diseases Therapeutics
The cost of treatment associated with Lysosomal Storage Diseases is very high. As it is an expensive method, this can restrain the growth of the global lysosomal storage diseases therapeutics market . For instance, according to an article published by Orphanet Journal of rare diseases on March 25, 2021, the enzyme replacement therapy is most effective therapy for treatment of lysosomal storage diseases. Hence research and development activities were more focused on it after initial success of ERT for Gauchers disease. Important limitation of many of the existing or pending therapeutic approaches is their expense. ERTs can cost anywhere from US$ 250– US$ 650,000/year per patient and gene therapies cost millions of dollars that are likely to be paid out over several years. These high costs are justified by the research and development costs incurred by the drug companies, the cost saving value of the treatments when compared to the medical costs of caring for these patients in the absence of these therapies and the very small commercial market for these disorders, especially when the drugs are being developed for individual diseases.
This restrain can be overcome by the introduction of newer government reimbursement policies that can help parents financially.
Geographic dispersal of lysosomal disease
The geographic dispersal of LSD hampers efforts to understand the disease, given that certain rare diseases are even rarer in some regions. For instance, according to article published by National Library of Medicine on July 25, 2022, both ethnicity and geography play a part in the incidence of LSDs. For example, Gaucher disease(GD) occurs in 1 in 40000 to 1 in 60000 in the general population. In Eastern Europen Jews (Ashkenazi Jews), GD is as high as 1 in 800, Tay-Sachs disease as high as 1 in 3900. Niemann-Pick A and mucolipidosis IV also occur with increased frequency in this population. In the Finnish population, aspartylglucosaminuria occurs at a frequency of 1 in 18500. Salla disease is also common in the Finns. Cultural and geographical genetic contribute to this high incidence.
This can be overcome by arranging awareness programs and dedicated research and development activities to understand disease and its relation with genetics.
Global Lysosomal Storage Diseases Therapeutics Market - Key Players
Major players operating in the global lysosomal storage diseases therapeutics market include Shire plc, Pfizer, Inc., Sanofi, BioMarin Pharmaceutical Inc., Actelion Ltd., Raptor Pharmaceutical Corp., Protalix Biotherapeutics Inc., Quest Diagnostics, and Amicus Therapeutics, Inc.
*Definition: The deficiency of enzymes that causes a specific disease condition called lysosomal storage disease includes diseases such as Fabry Disease, Gaucher disease, lysosomal acid lipase deficiency, mucopolysaccharidosis, Hunter syndrome, and Pompe disease. Enzyme replacement therapy involves intravenous administration of enzymes in order to correct the deficiency of enzymes. Enzyme replacement is associated with fewer side effects than other treatment methods. Enzymes are obtained from sources such as human cells, animal cells, and recombinant DNA technology. Enzyme replacement, although does not provide permanent cure, helps prevents permanent damage to the body caused due to deficiency of a specific enzyme. Treatment consists of weekly or monthly doses depending upon the disease.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients