Lysosomal Acid Lipase Deficiency Treatment Market Size and Forecast – 2025 – 2032
The Lysosomal Acid Lipase Deficiency Treatment Market size is estimated to be valued at USD 420 million in 2025 and is expected to reach USD 975 million by 2032, exhibiting a compound annual growth rate (CAGR) of 12.8% from 2025 to 2032.
Market Overview
Lysosomal acid lipase (LAL) deficiency is a rare inherited condition in which the body is not able to produce enough lysosomal acid lipase (LAL) enzyme, which is an important enzyme for breakdown of fatty materials namely cholesteryl esters and triglycerides. This results in building up of a large amount of fatty material in body organs, which include spleen, liver, and gut. The deficiency of LAL causes two autosomal recessive disorders, Wolman disease (WD) and Cholesteryl Ester Storage Disease (CESD). LAL deficiency is caused due to the genetic mutation in LIPA gene, which results in decrease or loss of Lysosomal Acid Lipase (LAL) enzyme.
Key Takeaways
ERT holds dominant market share within treatment types due to established efficacy and broad regulatory approvals, driving the Lysosomal Acid Lipase Deficiency Treatment market size significantly. Gene therapy is emerging as the fastest-growing sub segment with potential for long-term disease modification.
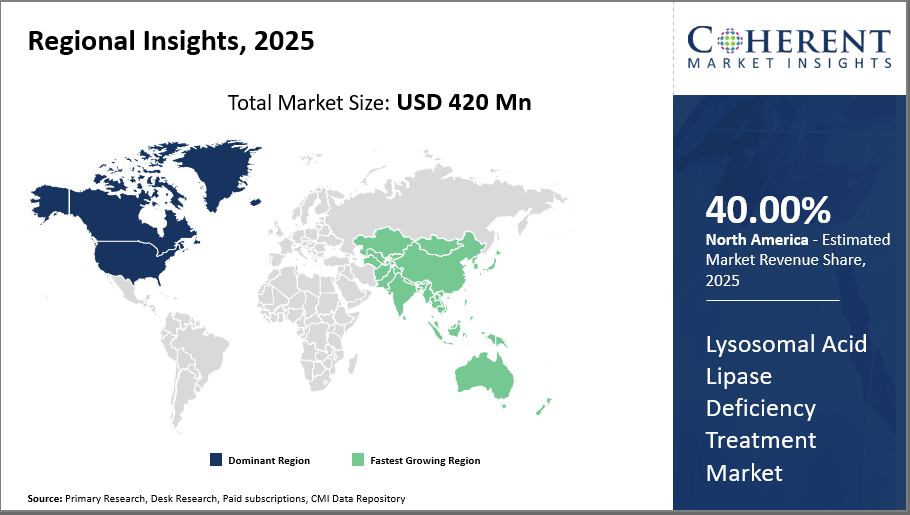
North America dominates the regional market, primarily attributed to robust healthcare infrastructure and early adoption, reflecting over 40% industry share in 2025. Meanwhile, Asia Pacific is the fastest-growing region, driven by increasing healthcare investments and disease awareness.
Lysosomal Acid Lipase Deficiency Treatment Market – Segmentation Analysis
To learn more about this report, Download Free Sample
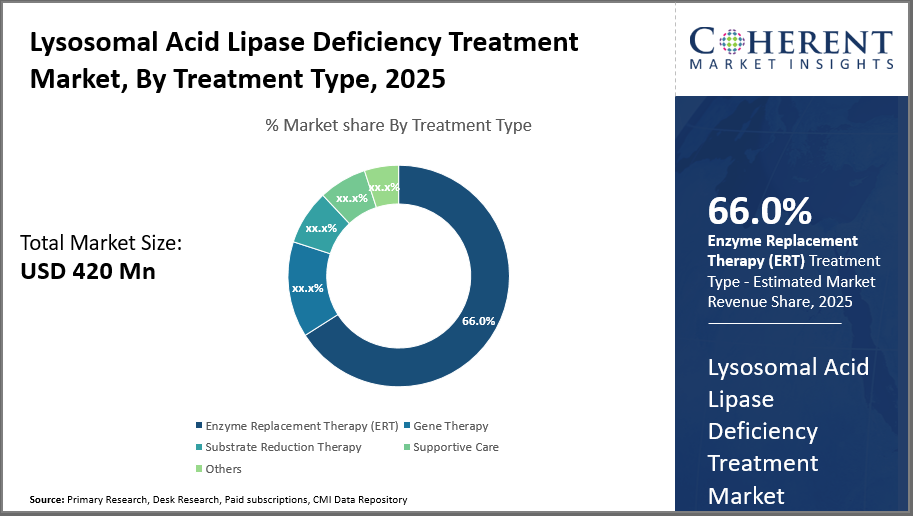
Lysosomal Acid Lipase Deficiency Treatment Market Insights, By Treatment Type
In terms of Treatment Type, Enzyme Replacement Therapy dominates the market share with 66%. This subsegment's dominance is driven by the established clinical efficacy and regulatory approvals of sebelipase alfa, which has become the treatment standard since FDA approval in 2015 and subsequent expansions. ERT improvements focus on dosing efficiency and immunogenicity reduction, sustaining its pivotal role.
Lysosomal Acid Lipase Deficiency Treatment Market Insights, By End User
Regarding End Users, Hospitals and Clinics dominate this segment, commanding a leading market share due to centralized administration of enzyme replacement therapies requiring infusion monitoring, accounting for over 50% of end user market share.
Lysosomal Acid Lipase Deficiency Treatment Market Insights, By Distribution Channel
In Distribution Channel segmentation, Hospital Pharmacies dominate with a 58% market share, favored by direct integration within healthcare delivery systems and bulk procurement benefits. Retail Pharmacies facilitate outpatient access but remain secondary due to specialty drug handling complexities.
Market Trends
The Lysosomal Acid Lipase Deficiency Treatment market is witnessing a shift toward personalized medicine approaches, particularly with gene therapy gaining traction as a potential curative option beyond conventional enzyme replacement therapy. In 2024, successful completion of Phase II gene therapy trials in the U.S. marked a paradigm shift in offering durable therapeutic benefits for LAL-D patients.
Furthermore, the integration of digital health platforms for patient monitoring and treatment adherence is growing, which improves the efficacy of therapies in the real world. Indicating growing diversifications beyond enzyme replacement, there is also an increase in investment in biotechnologies centred on substrate reduction therapy. These developments point to a future market ecology that will be influenced by digitally enabled care models and precision medicines, which will impact market growth strategies over the course of the next ten years.
Lysosomal Acid Lipase Deficiency Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Lysosomal Acid Lipase Deficiency Treatment Market Analysis amd Trends
In North America, particularly in the U.S., the Lysosomal Acid Lipase Deficiency Treatment market holds the largest industry share of over 40% attributed to favorable reimbursement policies, well-established healthcare infrastructure, and early adoption of enzyme replacement and gene therapies. The presence of major market players such as Alexion and Sanofi Genzyme fuels innovation and increases market share through robust clinical trial activities and patient assistance programs.
Asia Pacific Lysosomal Acid Lipase Deficiency Treatment Market Analysis and Trends
The fastest-growing region is Asia Pacific, with a compound annual growth rate (CAGR) of nearly 15%. This region's rise is fueled by government programs that facilitate access to rare illness treatments, growing healthcare spending, and increased disease awareness. Improved diagnostic infrastructure and growing patient populations are driving the market expansion of nations like China and India. Through partnerships, businesses such as Horizon Therapeutics have actively increased their footprint in this area, enhancing their market penetration.
Lysosomal Acid Lipase Deficiency Treatment Market Outlook for Key Countries
Acid Lipase Deficiency Treatment Trends in the United States
The United States remains a critical market due to its comprehensive healthcare infrastructure and leading position in rare disease research and treatment. The expansion of newborn screening programs across multiple states has reinforced early diagnosis rates, contributing to growth in market revenue.
Lysosomal Acid Lipase Deficiency Treatment Market Trends in Brazil
Strong healthcare regulations that prioritize managing rare diseases and robust reimbursement schemes are advantageous to the German market. Enzyme replacement medicines are now more accessible to people with LAL-D because to the government's backing of orphan medications and a well-functioning healthcare system.
Analyst Opinion
Increasing Adoption of Enzyme Replacement Therapies: Enzyme replacement therapy (ERT) remains the backbone treatment approach for LAL-D, with therapies such as sebelipase alfa driving substantial market revenue. In 2024, sebelipase alfa accounted for over 65% of the industry share due to efficacy and safety profiles documented in clinical settings worldwide. This adoption is underpinning the market growth trajectory as more countries incorporate ERT in standard treatment protocols.
Expansion of Diagnostic Capabilities Boosts Market Growth: The uptake of advanced molecular diagnostics for early LAL-D detection has intensified demand for targeted treatments, directly impacting market size. For example, healthcare systems integrating newborn screening programs, particularly in the U.S. and parts of Europe, witnessed a 22% increase in diagnosed cases in 2023 compared to 2022, translating into earlier intervention and increased treatment market revenue.
Growing Pipeline of Orphan Drugs Fuels Market Diversification: Several orphan drug candidates are advancing through clinical phases, diversifying treatment options beyond presently approved therapies. Notably, gene therapy candidates entering Phase II trials since 2024 show potential to reshape future market dynamics by offering durable treatment effects, which could alter competitive landscapes and stimulate industry share shifts.
Increasing Prevalence in Emerging Economies with Improved Healthcare Infrastructure: Regions including Asia Pacific and Latin America are recognizing LAL-D’s disease burden due to improved patient registries and growing healthcare expenditure. India’s government healthcare initiatives led to a 35% increase in market revenue from orphan drug usage for rare diseases including LAL-D in 2024, signifying expanding market scope.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 420 million |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 12.8% | 2032 Value Projection: | USD 975 million |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Alexion Pharmaceuticals, Sanofi Genzyme, Amicus Therapeutics, Audentes Therapeutics, Sangamo Therapeutics, Biomarin Pharmaceutical, RareGenix, Apellis Pharmaceuticals, Shire (Takeda), Moderna Therapeutics | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Lysosomal Acid Lipase Deficiency Treatment Market Growth factors
The rising incidence of LAL-D driven by increased disease awareness and enhanced diagnostic capabilities is a primary growth catalyst, evidenced by screening initiatives in countries like the U.S. and Germany.
Clinically validated therapies with favorable safety profiles such as sebelipase alfa are encouraging higher adoption rates, directly contributing to market revenue escalation.
Orphan drug designations and supportive regulatory frameworks are accelerating drug pipeline innovations, expanding treatment options and diversifying market scope.
Expanding healthcare infrastructure in Asia Pacific and Latin American regions is fostering greater patient access to advanced therapies, resulting in notable market share growth and business growth opportunities.
Lysosomal Acid Lipase Deficiency Treatment Market Development
In December 2024, the recent report from bioRxiv highlights, that the only enzyme known to break down triglycerides (TGs) and cholesterol esters (CEs) in lysosomes is lysosomal acid lipase (LAL). In the most extreme cases, LAL deficiency (LAL-D) can be a life-threatening illness in the early stages of infancy. It causes hepatosplenomegaly with a large buildup of CEs and TGs.
Key Players
Alexion Pharmaceuticals
Sanofi Genzyme
Amicus Therapeutics
Audentes Therapeutics
Biomarin Pharmaceutical
RareGenix
Apellis Pharmaceuticals
Shire (Takeda)
Moderna Therapeutics
To expand their LAL-D treatment portfolios, a number of top businesses are implementing creative expansion tactics, such as strategic alliances and licensing contracts. For example, Sanofi Genzyme's collaboration with pioneers in gene therapy sped up research and development, which resulted to successful early-stage clinical trials and increased market share in North America and Europe. In a similar vein, Alexion Pharmaceuticals improved patient access and increased regional income streams by implementing focused patient assistance programs throughout growing countries.
Lysosomal Acid Lipase Deficiency Treatment Market Future Outlook
Due to rising awareness and better diagnostic tools that allow for earlier and more precise patient identification, the market for treatments for lysosomal acid lipase deficiency has a bright future.
Improvements in enzyme replacement therapy, particularly targeted medications like sebelipase alfa, are improving patient survival and clinical results. Expanded new-born screening programs, orphan medicine designations, and growing government activities all contribute to market expansion.
In addition, it is anticipated that continued research and clinical trials for innovative therapies such as gene therapies will further transform available treatment options. Pharmaceutical companies, healthcare providers, and patient advocacy groups are working together to enhance accessibility and affordability, especially in emerging countries, despite obstacles such high treatment costs and restricted access in some areas.
The growing focus on AI-powered diagnostics and customized medicine also has the potential to revolutionize patient treatment in the field of rare genetic disorders, propelling the market's long-term expansion and innovation.
Historical Developments
In 2015, FDA approved genetically modified organisms (GMO) chickens for the production of a recombinant human lysosomal acid lipase (LAL) protein in their eggs for the treatment of lysosomal acid lipase (LAL) deficiency among Americans. Europe was expected to be the second largest region for the LAL deficiency treatment market. The European Commission in 2015 granted marketing permission of Kanuma for the treatment of LAL deficiency for patients of all ages, which was expected to fuel the growth of Kanuma in the region.
In 2015, FDA approved Kanuma, an innovative enzyme replacement therapy (ERT) in the U.S., European Union, and Japan, which provided an opportunity to manufacturers for exploring their business in the untapped market.
Sources
Primary Research interviews:
Pharmaceutical R&D heads
Patient advocacy group representatives
Databases:
ClinicalTrials.gov
Orphanet
GlobalData Healthcare Database
Magazines:
Genetic Engineering & Biotechnology News
The Pharma Letter
Rare Disease Report
Journals:
Molecular Genetics and Metabolism
Hepatology
Journal of Hepatology
Newspapers:
Associations:
Genetic Alliance
European Organisation for Rare Diseases (EURORDIS)
International Rare Diseases Research Consortium (IRDiRC)
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients