Lyophilization is also referred to as freeze-drying process. It is a method of isolating a solid substance from a solution by freezing the solution and evaporating it in a vacuum. The lyophilization process is predominately practiced in the biotechnological and pharmaceutical manufacturing industries for the manufacturing of good-quality products with a greater lifespan. Lyophilization is broadly used for the preservation of unstable and heat-sensitive substances and compounds such as microorganisms, proteins, antibodies, biologics, enzymes, blood plasma, vaccines, parenteral, diagnostic reagents, and nutraceuticals.
The global lyophilized product market is estimated to be valued at US$ 393.8 billion in 2021 and is expected to exhibit a CAGR of 7.3% during the forecast period (2021-2028).
Figure 1. Global Lyophilized Product Market Share (%) in Terms of Value, By Drug Class, 2021
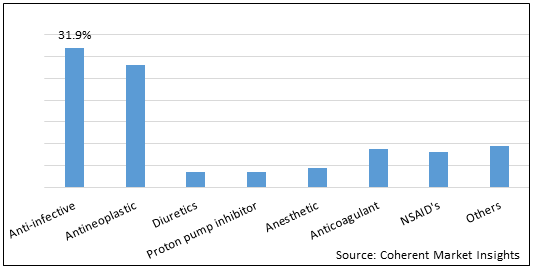
To learn more about this report, Download Free Sample
The increasing prevalence of infectious and autoimmune diseases is expected to drive the market growth during the forecast period
Increasing prevalence of infectious and autoimmune diseases is expected to drive the global lyophilized product market growth over the forecast period. For instance, according to an article published in Microbial Cell in 2020, annually, over 150 million severe cases of fungal infections occur worldwide, resulting in approximately 1.7 million deaths per year.
Lyophilized Product Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2020 | Market Size in 2021: | US$ 393.8 Bn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2021 to 2028 |
| Forecast Period 2021 to 2028 CAGR: | 7.3% | 2028 Value Projection: | US$ 645.1 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Sanofi, Merck & Co., Inc., Fresenius Kabi AG, Ciron Drugs & Pharmaceuticals Pvt. Ltd., Glaxosmithkline Plc, Mylan NV., Pfizer, Inc., Amneal Pharmaceuticals LLC, Otsuka Holdings Co. Ltd., BTG PLC, Bristol-Myers Squibb Company, Novartis AG, Takeda Pharmaceutical Company Limited, Biogen Inc., Dr. Reddy's Laboratories Ltd., Zydus Cadila, AbbVie Inc., Cipla Inc., Johnson & Johnson Services, Inc., Affy Pharma Private Limited, Jubilant Pharmova Limited, and Biocon |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 2. Global Lyophilized Product Market Share (%), By Packaging Type, 2021
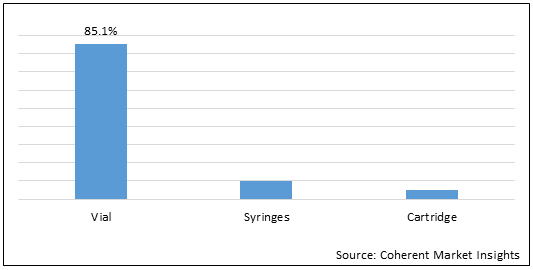
To learn more about this report, Download Free Sample
Increasing product launches and approvals are expected to drive the market growth during the forecast period.
Increasing product launches and approvals for lyophilized products are expected to drive the global lyophilized product market growth over forecast period. For instance, in May 2021, Dr. Reddy’s Laboratories Ltd., an Indian multinational pharmaceutical company, announced the launch of Ertapenem for Injection, 1 g/vial, a therapeutic equivalent generic version of INVANZ (ertapenem for injection) for injection, 1 g/vial approved by the U.S. Food and Drug Administration (USFDA) in the U.S. market
Global Lyophilized Product Market – Impact of Coronavirus (COVID-19) Pandemic
The coronavirus (COVID-19) pandemic and subsequent lockdown in various countries across the globe have impacted the financial status of businesses in all sectors. Private healthcare sector is one of the sectors which is majorly impacted by the COVID-19 pandemic. The coronavirus pandemic has also negatively impacted the development, production, and supply of medicines and the growth of the pharmaceutical businesses of various companies across the globe. The lockdown has resulted in the closure of industrial establishments, except the manufacturing of essential commodities, and disruption in the supply chain of pharmaceuticals and kits for diagnostic and therapeutic use. Thus, the COVID-19 pandemic has affected the economy in three main ways: 1) by directly affecting the production and demand; 2) disrupting distribution channels; and 3) causing financial impact on firms and financial markets. Supply chain and manufacturing activities in India, China, and the U.S. are disrupted due to global lockdown, while many countries such as Saudi Arabia, UAE, Egypt, and others are facing problems with regards to the transportation of drugs from one place to another
Global Lyophilized Product Market: Restraint
The major factors that are expected to hinder the global lyophilized product market growth include product recalls. For instance, in September 2021, Eli Lilly and Company is an American pharmaceutical company., voluntarily recalled lot D239382D, Expiration April 2022, of Glucagon Emergency Kit for Low Blood Sugar (Glucagon for Injection, 1 mg per vial; Diluent for Glucagon, 1 mL syringe), to the consumer/user level. The company recalled lot D239382D to the patient level because of a product complaint reporting that the vial of Glucagon was in liquid form instead of the powder form. The firm’s investigation indicates that the liquid in the Glucagon vial could be related to the manufacturing process. The use of the liquid form of this product may fail to treat severe low blood sugar due to the loss of potency
Key Players
Major players operating in the global lyophilized product market include Sanofi, Merck & Co., Inc., Fresenius Kabi AG, Ciron Drugs & Pharmaceuticals Pvt. Ltd., Glaxosmithkline Plc, Mylan NV., Pfizer, Inc., Amneal Pharmaceuticals LLC, Otsuka Holdings Co. Ltd., BTG PLC, Bristol-Myers Squibb Company, Novartis AG, Takeda Pharmaceutical Company Limited, Biogen Inc., Dr. Reddy's Laboratories Ltd., Zydus Cadila, AbbVie Inc., Cipla Inc., Johnson & Johnson Services, Inc., Affy Pharma Private Limited, Jubilant Pharmova Limited, and Biocon
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients