Global Lyme Disease Treatment Market Overview
Lyme disease is an infectious disease, caused due to the bacterium – Borrelia burgdorferi, which is transmitted to humans through bites of infected adult black-legged ticks. Most human infections are caused by bites of nymphs (immature tick). Ticks can attach to any part of the human body and can transmit the infection, if attached to the host body for 36 to 48 hours. Blacklegged ticks are responsible for spreading Lyme disease in the northeastern, mid-Atlantic, and north-central areas of the U.S. However, western black-legged ticks spread Lyme disease in the regions along the Pacific Coast. These regions are thus, expected to witness rapid growth of the market for Lyme disease treatment in the near future.
The early symptoms associated with Lyme disease include fever, headaches, chills, fatigue, muscle and joint aches, swollen lymph nodes, and characteristic skin rash (erythema migrans). According to the Centers for Disease Control and Prevention (CDC), erythema migrans affects around 70% to 80% of infected people, worldwide. However, untreated Lyme disease can lead to further discomfort, which includes intermittent pain in muscles, tendons, joints, and bones, coupled with severe headaches, neck stiffness, arthritis with severe joint pain, swelling in knees, facial palsy, increased heart palpitations or an irregular heartbeat, nerve pain, and inflammation of the brain and spinal cord.
Antibiotics are found to be the most effective treatment for Lyme disease. Patients with early stages of Lyme disease recover rapidly and completely with appropriate antibiotics such as doxycycline, amoxicillin, and cefuroxime axetil. Patients with certain neurological or cardiac illness may be treated with intravenous ceftriaxone. Patients bearing chronic symptoms of Lyme disease for over 6 months, can recover within a few weeks, through oral antibiotic treatment.
Lyme Disease Treatment Market Size and Forecast – 2025 – 2032
The Lyme Disease Treatment Market size is estimated to be valued at USD 1.45 billion in 2025 and is expected to reach USD 2.30 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 6.8% from 2025 to 2032.
Key Takeaways
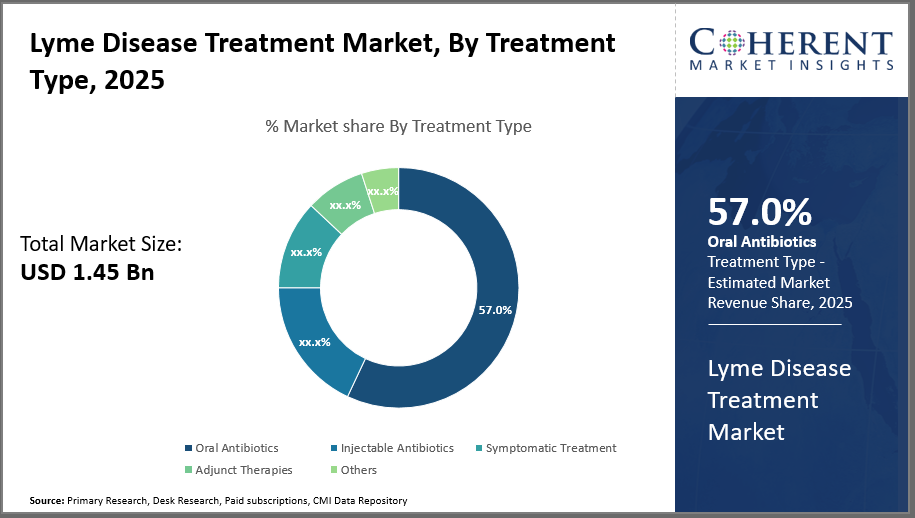
The oral antibiotics segment dominates the Lyme Disease Treatment market, holding 57% of industry share, driven by effective early-stage treatment protocols. Injectable antibiotics show the fastest growth, reflecting increasing complexities in late-stage Lyme disease management.
Hospitals remain the primary end user, accounting for over 60% of treatment administration, thanks to advanced healthcare infrastructure and trained professionals. Ambulatory and home care settings are increasingly growing, driven by patient preference for outpatient treatment.
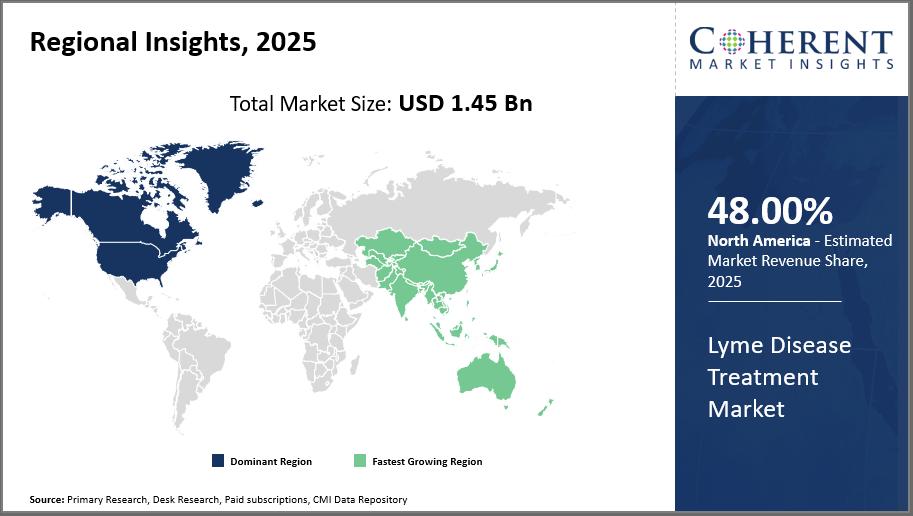
North America leads the market with more than 48% industry share, supported by proactive healthcare policies, extensive payer coverage, and significant disease prevalence. Asia Pacific is the fastest-growing region, presenting a CAGR of over 8%, fueled by improved disease awareness, expanding healthcare investment, and emerging economies.
Lyme Disease Treatment Market – Segmentation Analysis
To learn more about this report, Download Free Sample
Lyme Disease Treatment Market Insights, By Treatment Type
The market is divided into four segments based on the kind of treatment: oral antibiotics, injectable antibiotics, adjunct therapies, symptomatic treatment, and others. Because of their effectiveness in treating Lyme disease in its early stages and their ease of use, oral antibiotics hold a 57% market share among them.
Lyme Disease Treatment Market Insights, By Disease Stage
By Disease Stage, the market divides into Early-Stage Lyme Disease, Disseminated Lyme Disease, Late-Stage Lyme Disease, and Post-Treatment Lyme Disease Syndrome (PTLDS). Early-Stage Lyme Disease dominates the market, driven by higher incidence rates and effective oral antibiotic treatment options.
Lyme Disease Treatment Market Insights, By End User
The End User segmentation includes Hospitals, Specialty Clinics, Ambulatory Care Centers, Home Care Settings, and Others. Hospitals possess the largest market share due to their capability to administer complex intravenous treatments and manage severe Lyme disease cases.
Lyme Disease Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
Lyme Disease Treatment Market in North America: An Examination and Developments
In North America, dominance of the Lyme Disease Treatment market is driven by substantial industry share exceeding 48%, supported by well-established healthcare infrastructure, expansive diagnostic capabilities, and high disease awareness. Government initiatives and insurance coverage have further accelerated treatment adoption. Key companies such as Pfizer and Sanofi play influential roles, fortifying the market with new drug launches and enhanced distribution channels.
Lyme Disease Treatment Market in Asia Pacific: An Analysis and Advancements
Asia Pacific exhibits the fastest growth with an estimated CAGR above 8%. This surge is attributed to increasing cases due to climate change, improving diagnosis and treatment access, and rising healthcare expenditures in countries like China and India. The evolving regulatory landscape supporting approval of innovative antibiotics complements growing local manufacturing, positioning this region as a strategic growth hub.
Lyme Disease Treatment Market Outlook for Key Countries
Market Analysis and Trends for Lyme Disease Treatment in the United States
The USA represents the largest single-country market, driven by high incidence rates estimated at over 476,000 diagnosed cases annually according to CDC 2024 data. The presence of leading pharmaceutical players focusing on new antibiotic formulations and comprehensive public health programs bolsters the market. Reimbursement policies and increased investment in Lyme disease research further stimulate business growth and market revenue. Notable companies such as Pfizer and Johnson & Johnson maintain strong portfolios, frequently launching awareness campaigns and physician education programs to sustain demand.
Market Analysis and Trends for Lyme Disease Treatment in the Germany
Germany’s market is significant within Europe due to a strong healthcare system and rising Lyme disease cases reported by Robert Koch Institute in 2024. Emphasis on early diagnosis and treatment combined with government funding for infectious disease control drives market growth. Companies like Bayer AG and Roche Holding AG are engaged in clinical trials and market expansions. Germany’s regulatory environment facilitates the introduction of novel therapies, alongside increasing outpatient care adoption, shaping evolving market trends.
Analyst Opinion
The increasing demand for oral antibiotic therapies is a key quantitative driver in the Lyme Disease Treatment market. Oral antibiotics held approximately 57% of the market share in 2024, backed by their adaptability in early-stage Lyme disease treatment. Sales of doxycycline and amoxicillin have grown by 8.2% year-over-year (YoY) in North America, driven by primary care prescription trends.
Injectable antibiotic therapies, crucial for complicated or late-stage cases, exhibit a faster growth trajectory. This is supported by the rising prevalence of neuroborreliosis, which demands intravenous treatment. In Europe, hospital administrations reported a 12% increase in injectable antibiotic usage in 2024, demonstrating shifting treatment paradigms.
Diagnostic advancements such as the introduction of ELISA and Western blot tests have improved early detection rates by 15% over the past two years, reducing misdiagnoses and fueling demand for targeted treatments. For instance, the CDC’s 2025 reports indicated a 20% rise in appropriately diagnosed Lyme cases, directly impacting treatment market expansion.
The growing geriatric population, vulnerable to Lyme disease complications, further contributes to increasing treatment adoption. U.S. Medicare data from 2023 shows a 14% increase in Lyme disease treatment reimbursements, underscoring the demand-side market dynamics.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 1.45 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.8% | 2032 Value Projection: | USD 2.30 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Pfizer Inc., GlaxoSmithKline plc, Sanofi S.A., AbbVie Inc., Roche Holding AG, Johnson & Johnson, Novartis AG, Bayer AG, Merck & Co., Inc., Teva Pharmaceutical Industries Ltd. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Growth factors
The rapid expansion of the Lyme Disease Treatment market is propelled by several critical growth drivers. First, rising global prevalence of Lyme disease, particularly in temperate zones, has increased demand for effective treatment options, supported by CDC reports indicating over 476,000 diagnosed cases annually in the U.S. alone. Ongoing innovations in treatment formulations, including extended-release antibiotics and combination therapies, are enhancing patient outcomes and reducing relapse rates.
Expanding government funding and public health initiatives aimed at vector control and early diagnosis are fostering greater market penetration. Lastly, growing awareness campaigns targeting physicians and at-risk populations have contributed to improved clinical adoption rates and growing market revenue in recent years.
Lyme Disease Treatment Market Development
Market Trends
Recent years have seen a marked shift toward personalized Lyme disease treatment protocols integrating diagnostic biomarkers to customize antibiotic regimens. This trend is supported by advances in genomic and proteomic technologies, validated through clinical trials in 2024 that showed improved recovery rates by 18% with tailored therapy.
The market is witnessing shifts toward using adjunct symptomatic treatments such as anti-inflammatory agents and immunomodulators to address post-treatment Lyme disease syndrome (PTLDS), a move gaining evidence in rheumatology and infectious disease research.
Key Players
Pfizer Inc.
GlaxoSmithKline plc
Sanofi S.A.
AbbVie Inc.
Roche Holding AG
Johnson & Johnson
Novartis AG
Bayer AG
Merck & Co., Inc.
Teva Pharmaceutical Industries Ltd.
A number of prominent businesses have expanded their Lyme disease treatment portfolios through aggressive market growth tactics, such as strategic alliances and licensing contracts. To improve bioavailability and patient compliance, for instance, Pfizer and a biotech company that specializes in innovative antibiotic delivery methods signed a cooperative research and development partnership in 2024.
Outlook for the Lyme Disease Treatment Market in the Future
The market for treating Lyme disease has a bright future thanks to increased incidence rates, especially in North America and Europe, and growing global awareness. While environmental factors contribute to the disease's rising prevalence, government programs and regulatory incentives encourage the discovery of novel therapies. The market is anticipated to grow as a result of developments in diagnostic and therapeutic technologies, such as novel antibiotics and vaccine candidates.
Growth opportunities are also fueled by rising consumer demand, expanding healthcare access, and strategic collaborations in research and development. Emerging markets, especially in Asia-Pacific, are anticipated to contribute significantly to market expansion. Additionally, trends such as sustainability and regulatory support are expected to further enhance market growth, making it a key area of focus for investors and industry players in the coming years.
Historical Analysis
According to the Centers for Disease Control and Prevention (CDC), around 28,453 confirmed cases of Lyme disease and 9 new cases per 1,00,000 people were recorded in the U.S. during 2005–2015. The European market was expected to experience rapid growth in the Lyme disease treatment market in the near future. According to the 2014 factsheet of the European Centre for Disease Prevention and Control (ECDC), prevalence of Lyme disease was steadily increasing.
According to the National Surveillance Data, done by the National Notifiable Disease Surveillance System (NNDSS), between 2001 and 2010, Lyme carditis affected around 1% of patients suffering from Lyme disease in the U.S. Lyme carditis was treated with intravenous or oral antibiotics, preferably ceftriaxone. However, some patients required implantation of a temporary or permanent pacemaker, depending upon the severity of infection.
According to the International Lyme and Associated Disease Society (ILADS), Lyme disease was often difficult to diagnose and treat, resulting in persistent infections. ILADS recommended individualized treatment based on the severity of symptoms, tick-borne coinfections, and patient’s response to treatment. However, no single antibiotic or combination of antibiotics appeared to be capable of eliminating the Lyme disease infection. Therefore, the prevailing cases of Lyme disease and the large scope to develop the ideal antibiotic for treatment were expected to boost the Lyme disease treatment market in the near future.
The U.S. Food and Drug Administration (FDA), in July 2017, granted fast track designation to the first vaccine for Lyme disease – VLA15, created by Valneva SE – a Europe-based biotech firm, which was engaged in producing commercial vaccines for travelers. The company planned to initiate the phase 2 studies in early 2018. Introduction of the new vaccine was expected to further drive growth of the Lyme disease treatment market.
Sources
Primary Research interviews:
General physicians (GPs)
Pharmacists
Biotechnology researchers
Databases:
ClinicalTrials.gov
WHO Global Health Observatory
CDC WONDER Database
Magazines:
Pharma Times
The Scientist
BioWorld
Journals:
Journal of Infectious Diseases
Clinical Infectious Diseases
Emerging Infectious Diseases
Newspapers:
The Guardian (Global Health)
The Washington Post (Health & Science)
The Wall Street Journal (Pharma & Healthcare)
Associations:
International Lyme and Associated Diseases Society (ILADS)
American Society for Microbiology (ASM)
European Society of Clinical Microbiology and Infectious Diseases (ESCMID)
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients