Lumbar Disc Replacement Device Market is estimated to be valued at USD 1,570.8 Mn in 2025 and is expected to reach USD 3,858.7 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 13.7% from 2025 to 2032.
Analysts’ Views on Global Lumbar Disc Replacement Device Market:
Increasing advancements in products and ongoing research by key players and the high incidence of spinal cord injuries due to the increase in road accidents is expected to drive the growth of the global lumbar disc replacement device market. Whereas, an increase in the number of trauma-related and sports-related injuries, as well as orthopedic procedures is also expected to fuel the growth of the market over the forecast period. For instance, in June 2021, according to the Johns Hopkins University in the U.S., about 30 million children and teens participate in some form of organized sports, and each year, more than 3.5 million injuries occur that result in some loss of participation time for the participants. Almost one-third of all injuries incurred in childhood are sports-related injuries.
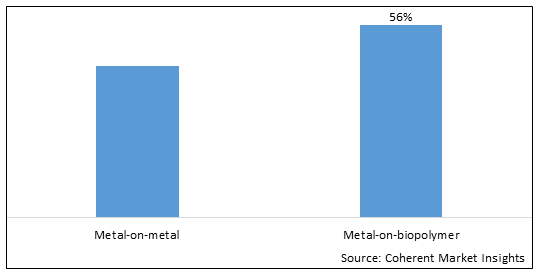
Figure 1. Global Lumbar Disc Replacement Device Market Share (%), By Material, 2025
To learn more about this report, Download Free Sample
Global Lumbar Disc Replacement Device Market– Drivers
New product launches by the key market players
New product launches by the key market players to drive the adoption of spinal devices is expected to drive the market over the forecast period. For instance, in July 2021, Orthofix US LLC., a global medical device company with a spine and orthopedics focus, announced the launch and first patient implants with the fiberFUSE Strip, an advanced demineralized fiber bone-graft solution containing cancellous bone. The fiberFUSE Strip is formulated as a convenient preformed bone-graft strip to enable optimized application for posterior cervical, posterior lumbar, and degenerative spinal procedures.
Technological advancements and strong research and development activities
Technological advancements and strong research and development activities are anticipated to drive the global lumbar disc replacement device market. For instance, in June 2020, Aesculap, Inc., a healthcare service provider offers an advanced product, named activL Artificial Disc. This is an ultra-high molecular weight lumbar disc device made of polyethylene that allows translational, and combines natural motion with mechanical stability, and rotational movement.
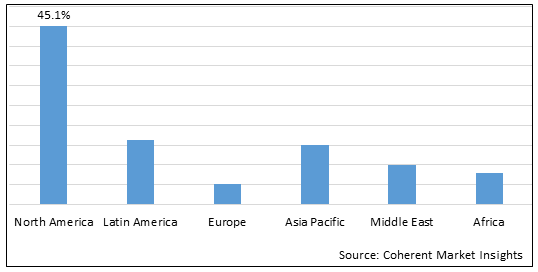
Figure 2. Global Lumbar Disc Replacement Device Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Global Lumbar Disc Replacement Device Market- Regional Analysis
Among region, North America is estimated to hold a dominant position in the global lumbar disc replacement device market over the forecast period owing to the increasing prevalence of lower back pain is expected to boost the growth of the lumbar disc replacement device market. For instance, in September 2025, according to the Spinal Cord Injury Report data, in 2020, it was estimated that the incidence of spinal cord injury (SCI) was around 48 cases per 1 million population in the U.S. In the same year, the prevalence of SCI was estimated to be around 291,000 patients in the U.S.
Global Lumbar Disc Replacement Device Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others faced problems regarding transportation of drugs from one place to another.
However, the COVID-19 pandemic had a negative impact on the global lumbar disc replacement device market. The spread of coronavirus is negatively impacting the sales of the global lumbar disc replacement device market which are majorly used for spine surgeries as many surgeries were canceled due to the COVID-19 pandemic. For instance, in July 2022, according to an article published by the Life Science Intelligence, Inc., a Saler of a consulting and market intelligence provider for the medical technology industry decreased by less than 13% in 2020 to just about US$7.2 billion. The necessity for in-patient recuperation was lessened by new technologies, which also shortened or times and simplified spine procedures. Ambulatory surgical facilities could be used for procedures, blunting the impact of COVID-19 limits in hospitals These technological gains are expected to have a lasting effect and drive future sales in this segment.
Lumbar Disc Replacement Device Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1,570.8 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 13.7% | 2032 Value Projection: | USD 3,858.7 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
AxioMed LLC., Medtronic, B. Braun SE, DePuy Synthes, NuVasive, Inc., Paradigm Spine LLC., Zimmer Biomet, Centinel Spine, LLC., Aesculap, Inc., Orthofix US LLC., and SYNERGY SPINE SOLUTIONS INC. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Lumbar Disc Replacement Device Market- Segmentation
The global lumbar disc replacement device market report is segmented into material, end user, and region.
Among Material, the global lumbar disc replacement device market is segmented into metal-on-metal and metal-on-biopolymer. Out of which, the metal-on-biopolymer segment is expected to dominate the market during the forecast period and this is attributed to better compatibility than the metal-on-metal, flexible movement of the spine, easy insertion, and high degree of rotation.
Among End User, the global lumbar disc replacement device market is segmented into hospitals and ambulatory surgical centers. Out of which, the hospitals segment is expected to grow in the global lumbar disc replacement device market over the forecast period, and this growth is attributed to the increasing patient population of lower back pain across the world as well as increase in the road accidents.
Among Region, the global lumbar disc replacement device market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa. Out of which, North America is expected to dominate the market over the forecast period owing to the increase in spinal cord injuries caused due to road accidents and the rising prevalence of people undergoing disc replacement surgeries propels the market growth.
Among all segmentation, the metal-on-biopolymer segment has the highest potential due to the increasing product launch by the key market players over the forecast period. For instance, in October 2022, DePuy Synthes, an orthopedic company part of Johnson & Johnson MedTech, announced that it has secured 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its TELIGEN System, an integrated technology platform that enables minimally invasive surgical transforaminal lumbar interbody fusion (MIS-TLIF) procedures through digital tools for visualization and access. The TELIGEN System is comprised of a tower that delivers a suite of technologies, including a camera control system, a VueLIF-T Procedure Kit with a disposable HD camera, a TELIGEN Clear Discectomy Device, and patient-based disposable ports. The TELIGEN System integrates with the UNLEASH bundle of implant solutions, which is designed to streamline the main stages in MIS-TLIF.
Global Lumbar Disc Replacement Device Market- Cross Sectional Analysis
Among material, the metal-on-biopolymer segment held a dominant position in North America region over the forecast period due to the increasing product launch by the key market players. For instance, in March 2021, Osseus Fusion Systems, a medical device company focused on developing advanced technology products announced the official launch of Aries-TC, its 3D printed transforaminal curved interbody fusion device. Aries devices are constructed from highly porous, 3D printed titanium optimized for bone fusion and biological fixation using PL3XUS, Osseus’ proprietary 3D-printing technology. PL3XUS titanium technology utilizes powder bed fusion, specifically Selective Laser Melting (SLM), to create a three-dimensional diamond lattice network with roughened surface topography designed to promote bony fusion onto, into, and throughout the device. The Aries-TC lumbar interbody fusion device comes in a wide variety of footprints, heights, and lordotic angles, to accommodate wide a variety of patient anatomies.
Global Lumbar Disc Replacement Device Market- Key Developments
In November 2022, Spinal Stabilization Technologies, Ltd., a developer of novel technologies & techniques for treating patients with lumbar discogenic back pain, announced the start of the LOPAIN2 clinical trial of the PerQdisc Nucleus Replacement Device (NRD). Spinal Stabilization Technologies, Ltd., PerQdisc Nucleus Replacement Device will be studied in patients with degenerative disc disease (DDD) of the lumbar spine, which causes severe back pain, in patients without stenosis or instability. The PerQdisc is used for surgical replacement of a single nucleus pulposus between spinal lumbar discs L1-S1 using an anterior or lateral transpsoas approach. Currently, there is no good surgical option for these patients. SST's PerQdisc NRD aims to treat discogenic low back pain while maintaining disc height and preserving range of motion.
On May 23, 2023, SYNERGY SPINE SOLUTIONS INC, an innovative orthopedic medical devices developer focused on artificial cervical disc replacement, announced a distribution partnership with LifeHealthcare, a medical device distributor, to expand access to the company’s marquis technology, the Synergy Disc, in Australia and New Zealand. The Synergy Disc is the only artificial cervical disc with a 6° lordotic core that combines cervical alignment and balance with the natural motion for patients with degenerative disc disease (DDD) causing radiculopathy and/or myelopathy. The distribution agreement builds on both companies’ shared values of providing surgeons in Australia and New Zealand with the latest medical device technologies along with increased focus and support.
On June 19, 2023, Orthofix US LLC, a global spine and orthopedics company, announced the full commercial launch of the WaveForm an interbody for Anterior Lumbar Interbody Fusion (ALIF) procedures. The WaveForm an interbody seamlessly integrates with the company’s Meridian ALIF system for treating patients in need of fusion due to degenerative disc disease. The proprietary wave-like design of the WaveForm an interbody provides a balance of strength, porosity and stability, with a large implant graft aperture for bone graft material to aid in creating an osteoinductive environment to optimize supplemental xation procedures.
On January 18, 2023, Centinel Spine, LLC, a global medical device company addressing cervical and lumbar spinal disease through anterior surgical access, announced that utilizing a new Current Procedural Terminology (CPT) code when performing two-level lumbar total disc arthroplasty via an anterior approach. To increase the availability of two-level lumbar total disc replacement (TDR) to individuals experiencing degeneration of the intervertebral discs, the American Medical Association (AMA) accepted the addition of a new Category I CPT code for a second level of lumbar TDR in late 2021. Centinel Spine's Prodisc L is the only total disc replacement system in the U.S. approved for two-level use in the lumbar spine.
Global Lumbar Disc Replacement Device Market- Key Trends
Acquisition by the key players
Acquisition by the key players to increase the product portfolio by increasing technological advancements is expected to drive the market over the forecast period. For instance, in February 2021, NuVasive, Inc., the company focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, announced that it has acquired Simplify Medical, a privately held company, and developer of the Simplify Lumbar Artificial Disc (Simplify Disc) for lumbar total disc replacement (cTDR). The acquisition of Simplify Medical adds the most clinically effective cTDR technology and further distinguishes NuVasive’s Lumbar portfolio in the market.
Global Lumbar Disc Replacement Device Market: Restraints
High cost of spinal surgeries
High cost of spinal surgeries is expected to hinder the market growth over the forecast period. So, to avoid this, Physical therapy is one of the cost-affordable for avoiding the spinal surgeries. For instance, in June 2020, according to an article published in the Journal of Wolters Kluwer, the average costs in the U.S were about US$ 14,000 for a single-level anterior cervical discectomy and fusion (ACDF) procedure and US$ 26,000 for a single-level (PLF) posterior lumbar fusion.
Global Lumbar Disc Replacement Device Market- Key Players
Major players operating in the global lumbar disc replacement device market include AxioMed LLC., Medtronic, B. Braun SE, DePuy Synthes, NuVasive, Inc., Paradigm Spine LLC., Zimmer Biomet, Centinel Spine, LLC., Aesculap, Inc., Orthofix US LLC., and SYNERGY SPINE SOLUTIONS INC.
Definition: Lumbar disc replacement is a type of spine surgery that involves the replacement of a damaged or degenerated disc of the spine with an artificial lumbar disc replacement device in the lumbar region. Lumbar disc replacement helps treat lower back pain. Currently, lower back pain is a major issue across the world; it may be acute, sub-acute, or chronic. Lumbar disc replacement devices are intended to help restore the natural distance between two vertebrae and the natural motion of the lumbar spine.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients