Low molecular weight heparin (LMWH) has several advantages over other heparin such as longer and more predictable activity than unfractionated heparin (UFH) and can be self-administrated at home via subcutaneous injection, reducing or eliminating hospital stays, thereby no regular blood monitoring required. Furthermore, heparins do not cross the placenta or harm the fetus, so they are the preferred anticoagulants for pregnant women who experience or who are at heightened risk of – blood clots. These advantages make LMWH as the most preferred heparin in inhibiting clotting factors.
The global low molecular weight heparin market is estimated to be valued at US$ 3,903.1 million in 2022 and is expected to exhibit a CAGR of 6.7% during the forecast period (2022-2030).
Figure 1. Global Low Molecular Weight Heparin Market Share (%), by Drug, 2022
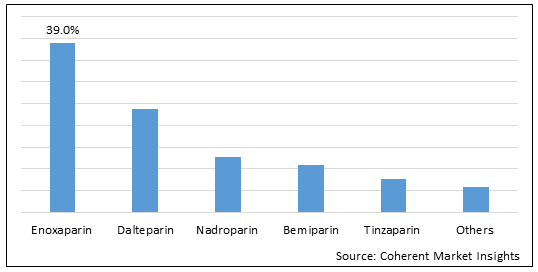
To learn more about this report, Download Free Sample
Increasing advantages of low molecular weight heparin over unfractionated heparin is expected to drive the market growth during the forecast period
Unfractionated heparin or standard heparin varies in action from patient to patient. Thus, it can only be administered to hospitalized patients under monitoring, while low molecular weight heparins (LMWHs) can be used subcutaneously once a day, without requirement for monitoring. Furthermore, LMWHs have more predictable pharmacokinetic properties as compared to unfractionated heparin (UFH), which allows LMWHs to be administered in fixed doses and without the need for dose adjustment based on laboratory monitoring.
The mean molecular weight of LMWH fractions is around 3,500–8,000 daltons, as compared with 15,000 daltons in unfractionated material. As low-molecular-weight fractions of heparin react less with platelets than high-molecular-weight fractions, it was also expected that LMWH would less often induce immuno-allergic thrombocytopenia, a severe side-effect of UFH that is often complicated by arterial thrombosis. Such advantages of low molecular weight heparin over unfractionated heparin are driving growth of the low molecular weight heparin market. Furthermore, properties such as better bioavailability, predictable dose response, and longer plasma half-life than unfractionated heparin, makes low molecular weight heparin a better candidate for anticoagulant therapy. This in turn is fuelling demand for low molecular weight heparin, and thus it is expected to boost the market growth over the forecast period.
Low Molecular Weight Heparin Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 3,903.1 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 6.7% | 2030 Value Projection: | US$ 6,539.3 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Pfizer, Inc., LEO Pharma A/S, Sanofi S.A., Teva Pharmaceutical Industries Ltd., Amphastar Pharmaceuticals Inc., Abbott Laboratories, Aspen Pharmacare Holdings, Laboratorios Farmaceuticos ROVI SA, Changzhou Qianhong Biopharma, and Intrapharm Laboratories. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 2. Global Low Molecular Weight Heparin Market Share (%), by Region, 2022
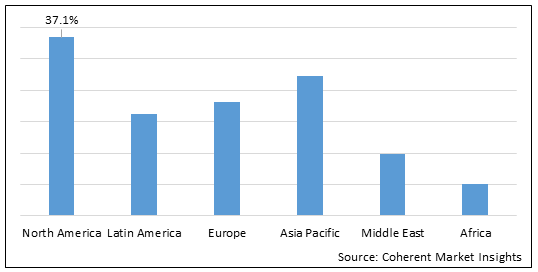
To learn more about this report, Download Free Sample
Increasing introduction of biosimilar low molecular weight heparin anticoagulants globally is expected to aid in the market growth
Low molecular weight heparin usage is expected to increase over the forecast period, due to a number of other non-anticoagulant properties. These include anti-tumor, anti-inflammatory, and anti-proliferative actions (in pathologies such as nephrotic syndrome and Alzheimer’s disease). The patents of low molecular weight heparins have now expired. However, the market potential for LMWHs is witnessing growth, owing to increasing use in a number of Western European countries and emerging economies and its potential indications, such as thromboprophylaxis, Venous thromboembolism (VTE), anticoagulation treatment during pregnancy, among others. Therefore, pharmaceutical companies in many countries are focused on producing generic forms of Low Molecular Weight Heparin.
Pharmaceutical companies produced either as biosimilars or named generic versions of the branded LMWH. Specifically, copies of enoxaparin are available in North and Latin America and Asian countries. In Australia and Europe, regulatory bodies developed specific requirements for approval of biosimilar LMWHs.
Furthermore, according to the article published by the National Center for Biotechnology Information, biosimilar low molecular weight heparins are competitive and less expensive than the originator. For instance, in July 2022, Teva Pharmaceuticals, pharmaceutical company, the global research and development organization collaborates to propel the company’s mission to be a global leader in generics and biopharmaceuticals. The Teva Pharmaceuticals is on mission of development to commercial launch of small molecules, novel biologics and biosimilars, our uniquely integrated “One Teva” drug development model combines our strength in generics with our knowledge of innovative drug development. Thus, Teva Pharmaceutical Industries Ltd. is offering the platform for many other research and development of biosimilar molecule for heparin which is expected to drive the global low molecular weight heparin market growth.
Global Low Molecular Weight Heparin Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization had declared it a public health emergency on January 30, 2020.
COVID-19 can affect the economy in three main ways: by directly affecting production and demand of drugs, prebiotics, probiotics, medical devices, etc. by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, U.A.E., Egypt, and others, are facing problems with regards to the transportation of drugs from one place to another.
However, the COVID-19 pandemic had positive impact on the global low molecular weight heparin market, owing to the increasing research and development activities for developing low molecular weight heparin for COVID-19 treatment. For instance, according to the data published by the National Center for Biotechnology Information, on August 03, 2022, meta-analysis carried out by the European Society of Cardiology showed that administration of low molecular weight heparin was associated with better outcomes compared with unfractionated heparin in hospitalized COVID-19 patients. Prospective cohorts and Randomized controlled clinical trials (RCTs) are urgently needed in exploring the definitive effect of low molecular weight heparin to provide direct high-certainty evidence.
Furthermore, according to the data published by the BioMed Central Ltd., Part of Springer Nature, in critical COVID-19 patients, usually admitted to the intensive care unit (ICU), high dose prophylactic heparin without a documented Venous thromboembolism is not recommended, whereas in hospitalized non-ICU patients it should be considered after having evaluated the bleeding risk. Prophylactic heparin at a high/intermediate dose did not reduce the primary outcome compared with the low dose but increased the risk of major hemorrhagic events. Thus, the key players in the market were engaged in research and development activities for the low molecular weight heparin.
Global Low Molecular Weight Heparin Market: Key Developments
In March 2022, Valeo Pharma Inc., pharmaceutical company based in Canada announced that it has successfully entered into a Product Listing Agreement (PLA) with the British Columbia Minister of Health, for the listing and public reimbursement of Redesca and Redesca HP(are the first LMWH biosimilars to be approved by Health Canada), its low molecular weight heparin ("LMWH") biosimilar for the prevention and treatment of thromboembolic disorders, on the British Columbia PharmaCare list of medications, effective on March 22, 2022.
Global Low Molecular Weight Heparin Market: Restraint
The major factors that hinder growth of the global low molecular weight heparin market include adverse effect of low molecular weight heparin. Heparin induced thrombocytopenia (HIT), an adverse reaction occurring during treatment with heparin, is associated with inconsistent increase in the clotting causing further complications. Heparin-induced thrombocytopenia (HIT) is an adverse reaction that can occur during treatment with heparin. Increasing epidemic of Heparin-induced thrombocytopenia (HIT) due to application of heparin is restricting the growth of market. For instance, according to the data published in American Society of Hematology in 2017, Heparin-induced thrombocytopenia (HIT) is heparin’s most clinically relevant non-hemorrhagic complication. Furthermore, adults receiving heparin formulation for medical or general surgical indications are at higher risk for HIT than pediatric or obstetric patients.
Key Players
Major players operating in the global low molecular weight heparin market include Pfizer, Inc., LEO Pharma A/S, Sanofi S.A., Teva Pharmaceutical Industries Ltd., Amphastar Pharmaceuticals Inc., Abbott Laboratories, Aspen Pharmacare Holdings, Laboratorios Farmaceuticos ROVI SA, Changzhou Qianhong Biopharma, and Intrapharm Laboratories.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients