Liver fibrosis is not a specific condition but rather a symptom of another liver problem. Liver fibrosis causes no symptoms itself. Healthcare professionals focus on the treatment of liver fibrosis by treating the cause of liver fibrosis, to stop or slow down the scarring of the liver. Chronic alcohol drinking, autoimmune hepatitis, storage and metabolism disorders such as Wilson disease, Galactosemia, Fructosemia, glycogen storage diseases, iron-overload symptoms, Gaucher disease, some of the bacterial, parasite and viral infections, disorders affecting hepatic blood flow, among others are some of the possible risk factors associated with liver fibrosis management. Furthermore, liver fibrosis treatment market is witnessing increased pace in the Non-alcoholic Steatohepatitis (NASH) treatment.
Global liver fibrosis treatment market is estimated to be valued at US$ 14.7 billion in 2022 and is expected to exhibit a CAGR of 10.8% during the forecast period (2022-2030).
Figure 1. Global Liver Fibrosis Treatment Market Share (%), by End User, 2022
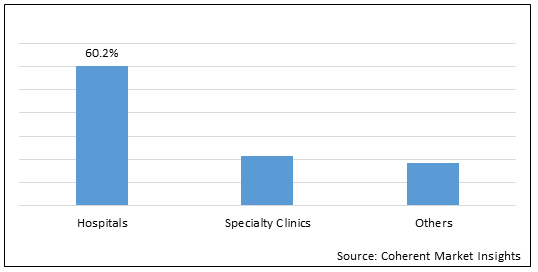
To learn more about this report, Download Free Sample
Funding initiatives by various private research organizations to conduct research related to development of treatment for liver fibrosis and other fatty liver diseases is expected to drive growth of the liver fibrosis treatment market.
Increasing research by various private research organizations to conduct research related to development of treatment for liver fibrosis and other fatty liver disease is expected to drive growth of the liver fibrosis treatment market. For instance, in August 2018, College of Science at Virginia Tech and Department of Pharmacology at the University of Virginia, based in the U.S. received US$ 400,000 grant from the Virginia Biosciences Health Research Corporation (VBHRC) with US$ 800,000 matching funds from Continuum Biosciences, Inc. This grant along with matching funds would help researchers to develop drugs to treat nonalcoholic steatohepatitis (NASH).
Liver Fibrosis Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 14.7 Bn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 10.8% | 2030 Value Projection: | US$ 33.4 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Gilead Sciences, Inc., Merck & Co., Inc., Bristol-Myers Squibb, Johnson and Johnson, Novartis AG, Vertex Pharmaceuticals Incorporated, Pfizer Inc., FibroGen, Inc., and Pharmaxis Limited. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 2.Global Liver Fibrosis Treatment Market Share (%), by Region, 2022
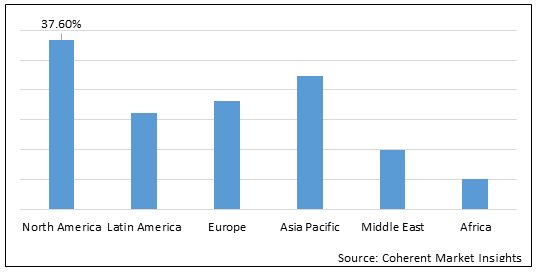
To learn more about this report, Download Free Sample
Key players in the liver fibrosis treatment market are engaged in pre-clinical and clinical studies to develop novel treatment therapies in liver fibrosis regime, which is expected to drive the market growth over the forecast period.
Increasing research and development activities by key players in pre-clinical and clinical studies to develop novel treatment therapies in liver fibrosis regime is expected to drive the global liver fibrosis treatment market growth. For instance, in March 2018, the U.S. Food and Drug administration granted Orphan Drug Designation for PTG-300: a subcutaneous injectable developed by Protagonist Therapeutics, Inc. The PTG-300 is currently in clinical development stage for the potential treatment of beta-thalassemia, and can also be used in the liver fibrosis treatment. Furthermore, Gilead Sciences, Inc., a biopharmaceutical company, in October 2017, released the Phase II result studies for Selonsertib: GS-0976 in Nonalcoholic Steatohepatitis (NASH) stage F3 liver fibrosis. These studies reported that, GS-0976 led to significant reductions in concentration of liver fat and fibrosis. Moreover, Gilead Sciences also possesses 18 other pipeline molecules in the liver fibrosis treatment, which is further expected to boost company’s revenue in 2025.
Global Liver Fibrosis Treatment Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization had declared it a public health emergency on January 30, 2020.
COVID-19 can affect the economy in three main ways: by directly affecting production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, U.A.E., Egypt, and others, are facing problems with regards to the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a negative impact on the global liver fibrosis treatment market, owing to the decrease in demand of drugs that are used in liver treatment. For instance, in January 2020, according to the data published by the National Center for Biotechnology Information, therapy of COVID-19 includes oral and parenterally administered agents, antiviral drugs, immunomodulatory medications, anticytokines, monoclonal antibodies, and miscellaneous agents. The main focus of the drug developers was to manufacture and the supply the drugs, which are included in treatment of COVID-19 pandemic. Vaccines effective in preventing COVID-19 were developed rapidly and approved for use by the FDA in the U.S. under emergency use authorization. Thus, demand of drugs indicated for the use in other disease conditions were manufactured in limited quantity as compared to those used in treatment of COVID-19.
Global Liver Fibrosis Treatment Market: Key Developments
In December 2021, Beijing Continent Pharmaceutical Co, Ltd., a pharmaceutical company, had carried out Phase II clinical trial successfully for Hydronidone capsules, and was promoted for phase 3 clinical trial in January 2022. The phase 3 clinical trial studies for Hydronidone capsules are still ongoing and expected to complete study of clinical trial in June 2024.
Global Liver Fibrosis Treatment Market: Restraint
The major factors that hinder growth of the global liver fibrosis treatment market include high cost required for drug development of liver disease treatment procedure. For instance, in January 2019, according to data published by PubMed, the race in the pharmaceutical industry to develop drugs to treat a form of fatty liver disease, industry experts estimate the global market for these new drugs is $35 billion. The U.S. spends US$5 billion annually in health-care costs related to the disease, which include chemotherapy, transplants, tests and hospitalizations, as reported by the Center for Disease Analysis.
Key Players
Major players operating in the global liver fibrosis treatment market include Gilead Sciences, Inc., Merck & Co., Inc., Bristol-Myers Squibb, Johnson and Johnson, Novartis AG, Vertex Pharmaceuticals Incorporated, Pfizer Inc., FibroGen, Inc., and Pharmaxis Limited.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients