Liver Diseases Therapeutics Market is estimated to be valued at USD 20.5 Bn in 2025 and is expected to reach USD 39.44 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 9.8% from 2025 to 2032.
Analysts’ Views on Global Liver Diseases Therapeutics Market:
Increasing prevalence of liver diseases, the launch of newer drug therapies for liver diseases by key market players, and increased research and development activities in liver disease therapeutics are expected to boost the growth of the global liver diseases therapeutics market over the forecast period. For instance, according to data published by the American Liver Foundation on August 5, 2022, more than 100 million people in the U.S. have some form of liver disease. 4.5 million U.S. adults (1.8%) have been diagnosed with liver disease. While in 2020, 51,642 adults in the U.S. died from liver disease. Chronic liver disease was the 12th leading cause of death in the U.S. in 2020
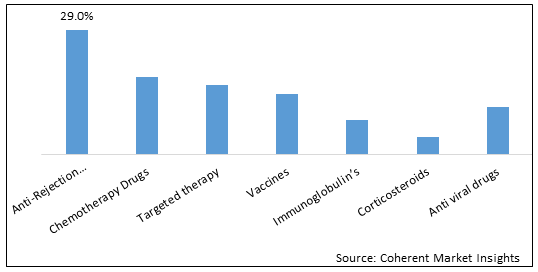
Figure 1. Global Liver Diseases Therapeutics Market Share (%), By Therapy Type, 2025
To learn more about this report, Download Free Sample
Global Liver Diseases Therapeutics Market – Driver
Approval of newer drugs by regulatory bodies for treatment of liver diseases
The increasing approval by the U.S. Food and Drug Administration for the treatment of liver diseases is expected to drive the growth of the global liver diseases therapeutics market over the forecast period. For instance, on May 29, 2020, U.S. Food and Drug Administration (FDA) approved atezolizumab (Tecentriq) and bevacizumab (Avastin) as an initial treatment for people with liver cancer that has spread or that can’t be treated with surgery. In the study that led to the approval, called IMbrave150, liver cancer patients treated with atezolizumab and bevacizumab lived substantially longer without their cancer getting worse than those treated with sorafenib (Nexavar).
Strategies like collaborations to introduce newer therapies in liver disease treatment
The increasing collaborations between key market players is expected to drive the growth of the global liver disease therapeutics market. For instance, on November 4, 2022, Alimentiv Inc., U.S. based biotechnology company, collaborated with Summit Clinical Research, U.S. based research institute, to tackle non-alcoholic steatohepatitis. According to research of carried by Alimentive Inc., there are over 100 drugs in clinical development. An improved clinical trial design, faster recruitment, reduced failure rate and improved patient experience can be achieved due to expertise of Summit clinical research institute as research organization in carring out clinical trials.
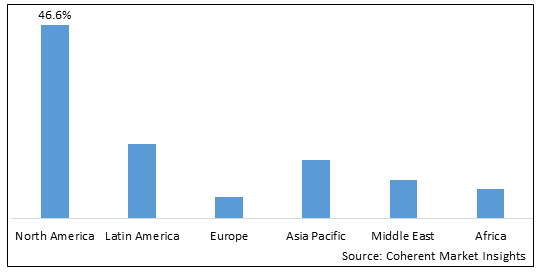
Figure 2. Global Liver Diseases Therapeutics Market Value (US$ Billion), By Region, 2025
To learn more about this report, Download Free Sample
Global Liver Diseases Therapeutics Market - Regional Analysis
Among regions, North America is estimated to hold a dominant position in the global liver diseases therapeutics market over the forecast period. This is due to the approval of newer combination therapies for the treatment of liver diseases by the U.S. FDA. For instance, on October 24, 2025, U.S. FDA approved a new immunotherapy combination for the treatment of hepatocellular carcinoma, the most common type of liver cancer. This combination consists of a single dose of tremelimumab (Imjudo) followed by treatment with durvalumab (Imfinzi) in a Single Tremelimumab Regular Interval Durvalumab (STRID) regimen.
Global Liver Diseases Therapeutics Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others are facing problems with the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a negative impact on the global liver diseases therapeutics market. This is because of reduced research activities during the COVID period. For instance, according to an article published by the journal Annals of Hepatology on May 18, 2020, clinical research unrelated to COVID-19 has been greatly altered during this pandemic, due to quarantines and concerns regarding patient and research team safety. The U.S. FDA issued guidelines for ensuring the safety of clinical trial participants and flexibility for amending clinical protocols, particularly for including alternatives to in-patient visits. Basic research has been similarly affected.
Liver Disease Therapeutics Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 20.5 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.8% | 2032 Value Projection: | USD 39.44 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Astellas Pharma Inc., Bristol-Myers Squibb, Gilead Sciences, Glaxosmithkline Plc, F. Hoffmann-La Roche Ltd., Merck & Co. Inc., Novartis AG, Sanofi S.A, Pfizer Inc., Takeda Pharmaceutical, Valeant Pharmaceuticals, Watson Pharmaceuticals, Inc., Theratechnologies Inc., Alnylam Pharmaceuticals, Inc., Protagonist Therapeutics, Inc., Dicerna Pharmaceuticals, Inc., Endo International, Provectus Biopharmaceuticals Inc., and MAX BioPharma, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Liver Diseases Therapeutics Market Segmentation:
The global liver diseases therapeutics market report is segmented into therapy type, distribution channel, and region
Based on Therapy type, the global liver diseases therapeutics market is segmented into anti-rejection drugs/immunosuppressants, chemotherapy drugs, targeted therapy, vaccines, anti-viral drugs, immunoglobulins, and corticosteroids. Out of which, immunosuppressants is expected to dominate the market due to increased use of immunosupressants in liver disease treatment.
Based on Distribution channel, the global liver diseases therapeutics market is segmented into hospital pharmacies, retail pharmacies, and online pharmacies. Out of which hospital pharmacies are expected to dominate the market due to the increased number of hospital pharmacies providing treatment medications.
Based on Region, the global liver diseases therapeutics market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East & Africa. Out of which North America segment is expected to dominate the market due to increased research and development activities.
Among all the segmentations, the therapy type segment has the highest potential due to the increasing approvals and launch of newer products by key market players. For instance, on November 2, 2022, U.S. FDA approved a supplemental new drug application (sNDA) for Vemlidy (tenofovir alafenamid) for treatment of chronic hepatitis B viral infection in pediatric patients. Vemlidy’s approval in this pediatric patient population is supported by 24-week data from a Phase 2 clinical trial comparing treatment with Vemlidy 25 mg to placebo among 70 patients aged 12-18 years and weight 35 kg.
Global Liver Diseases Therapeutics Market Cross Sectional Analysis:
Increase in the launch of drugs for diagnosis and treatment of liver diseases in Asia Pacific region is expected to drive the growth of the therapy type segment in this region. For instance, on May 24, 2023, Asahi Kasei Pharma, a Japan-based pharmaceutical company, announced that Doptelet Tablet 20 mg (avatrombopag maleate) obtained exclusive distribution rights in Japan for the treatment of severe thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo an invasive procedure from Swedish Orphan Biovitrum Japan Co., Ltd., Sweden based biopharmaceutical company. It has been listed on Japan’s National Health Insurance (NHI) drug price standard. The launch of the product was scheduled for June 1, 2023.
Global Liver Diseases Therapeutics Market: Key Developments
On August 5, 2022, Metadeq Diagnostics, U.K. based diagnostic startup, announced that it has developed a noninvasive blood test that can bypass the need for liver biopsies when diagnosing the severity of liver disease. The test was developed with collaborators from King's College, U.K. based research university, and it is intended to measure and stage nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), and liver fibrosis, which can eventually progress to cirrhosis of the liver.
On September 22, 2021, Mirum Pharmaceuticals Inc., U.S. based pharmaceutical company, entered into an agreement with Takeda Pharmaceuticals Co. Ltd, a Japan-based healthcare company for the development and commercialization of maralixibat in Japan for Alagille syndrome (ALGS), progressive familial intrahepatic cholestasis (PFIC), and biliary atresia (BA). Maralixibat is an investigational, orally administered medication. It is being evaluated globally for ALGS, PFIC, and BA.
On February 23, 2023, Predictive Health Intelligence Ltd., U.K. based medical technology company, launched hepatoSIGHT, a data-driven tool for the identification of undiagnostic liver disease using historic blood tests.
On September 19, 2022, Genfit, France based biotechnology company, announced that it had entered into an agreement to acquire Versantis AG, Switzerland-based pharmaceutical company, to expand its liver disease portfolio. The acquisition by Genfit is to become a global leader in acute on chronic liver failure. Genfit will also further expand its pipeline in other liver diseases characterized by high unmet medical needs with additional product candidates developed by Versantis.
Global Liver Diseases Therapeutics Market : Key Trends
Awareness programs by government and non-profit organizations can drive market growth. On March 30, 2022, Global Liver Institute, U.S. based nonprofit organization, launched its ‘Liver health is Public health’ initiative. It is a multi-year awareness program that will address ongoing challenges in the diagnosis and treatment of liver diseases, and shift the conversation on liver health. It will develop workgroups and assess the state of liver health globally, develop education resources for diverse audiences, and empower a global cohort of liver health advocates.
Introduction of newer therapies by researchers can drive market growth. On January 24, 2023, the Medical University of Vienna, Australia-based public research university, announced that it has developed a new liver disease treatment method for alcohol-related liver disease treatment. The researchers collaborated with a team from the University of California, U.S.-based research university. Together they identified a previously unknown function of the polymeric immunoglobulin receptor (pIgR) in alcohol-related liver disease. Preclinical studies have shown that pIgR-mediated secretion of certain antibodies (immunoglobulin A or IgA) in the intestines is vital in protecting the liver against alcohol-related damage. Hence, enhancing pIgR in the liver or increasing the IgA levels in the intestine could be a promising starting point for novel treatment options for alcohol-related liver disease.
Global Liver Diseases Therapeutics Market: Restraint
Availability of alternative methods to liver disease therapeutics
The treatment of chronic liver diseases lasts for a longer duration which makes it expensive and reduces patient adherence to treatment. The availability of alternative methods like organ transplantation and liver resection can limit the growth of the global liver diseases therapeutics market. For instance, according to an article published by the World Journal of Transplantation on March 31, 2020, different treatments like Xeno-organ transplantation, scaffold-based transplantation, and cell transplantation can be employed for the treatment of liver failure. Cell transplantation can be achieved using hepatocytes, umbilical cord-derived mesenchymal stem cells (MSCs), bone marrow-derived MSCs, and hematopoietic stem cells.
This counterbalance this restrain, a more effective treatment should be introduced with vigorous research and development activity that can minimize the need for organ transplantation.
Withdrawal of clinical trials
Many clinical trials are being conducted to ensure that the treatment is safe and effective. However, due to uninspiring results, the clinical trials are withdrawn from the market which can limit the growth of the global liver diseases therapeutics market during the forecast period. For instance, on October 25, 2022, Novartis AG, a Switzerland-based pharmaceutical company, announced that it has stopped work on the use of iscalimab for liver transplant program after the phase 2 trial delivered lackluster results. Phase 2 trial found the anti-CD40 antibody has a less favorable risk-benefit profile than tacrolimus, which Astellas Pharma sells as Prograf, in liver transplant patients
This can be counterbalanced by carrying out more dedicated research on newer drug therapies that can provide better results.
Global Liver Diseases Therapeutics Market - Key Players
Major players operating in the global liver diseases therapeutics market include Astellas Pharma Inc., Bristol-Myers Squibb, Gilead Sciences, Glaxosmithkline Plc, F. Hoffmann-La Roche Ltd., Merck & Co. Inc., Novartis AG, Sanofi S.A, Pfizer Inc., Takeda Pharmaceutical, Valeant Pharmaceuticals, Watson Pharmaceuticals, Inc., Theratechnologies Inc., Alnylam Pharmaceuticals, Inc., Protagonist Therapeutics, Inc., Dicerna Pharmaceuticals, Inc., Endo International, Provectus Biopharmaceuticals Inc., and MAX BioPharma, Inc.
Definition: Liver Diseases Therapeutics are used for the treatment of hepatitis A, hepatitis B, and hepatitis C; liver fibrosis, liver cancer; fatty liver disease and cirrhosis; hemochromatosis and Wilson disease. Increasing prevalence of liver diseases such as liver fibrosis is expected to propel the growth of the global liver diseases therapeutics market over the forecast period. Other factors influencing the growth of the market constitute of growing prevalence of risk factors such as chronic infection with hepatitis B or C virus, compromised immune system due to co-infection with HIV, or use of immunosuppressive drugs after a liver transplant.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients