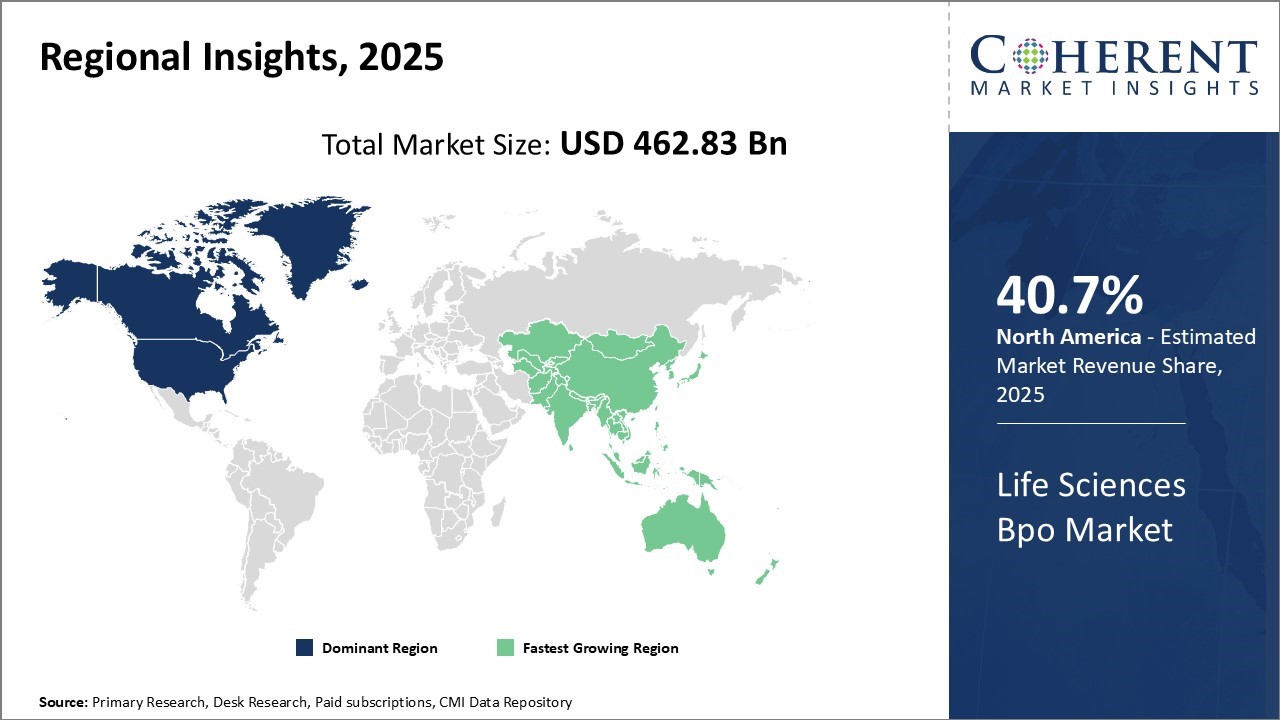
Global life sciences BPO market is estimated to be valued at USD 462.83 Bn in 2025 and is expected to reach USD 1,116.81 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 13.4% from 2025 to 2032.
To learn more about this report, Download Free Sample
Global life sciences BPO market growth is driven by increasing research and development expenditure globally and rising reliance on outsourcing of non-core functions by pharmaceutical and biotech companies. Growing awareness for cost control alongside focus on expansion of manufacturing capabilities and services has encouraged market players to outsource business processes to third-party life sciences BPO providers. Furthermore, life sciences BPO’s bring opportunities to leverage location and technology advantages from their global network to increase efficiencies and optimize costs. This helps research organizations to focus more on their core functions and pipelines.
|
Current Events |
Description and its impact |
|
US-China Tech Deceleration |
|
|
AI Adoption in Clinical Operations |
|
|
Drug Shortage Crisis Response |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The pharmaceutical outsourcing segment is projected to dominate the market with a 60.6% share in 2025 due to several key factors. Pharmaceutical and biotech companies increasingly outsource clinical trials and related services to Contract Research Organizations (CROs). CROs excel in managing comprehensive aspects of drug development, from early-stage research to post-marketing trials, leveraging their expertise in clinical monitoring, data management, biostatistics, and regulatory compliance. This specialization helps sponsors accelerate development timelines, efficiently navigate complex regulatory landscapes, and optimize resource allocation. By offering end-to-end solutions and access to global patient populations, CROs enhance trial quality and cost-efficiency, making them the preferred partners in the pharmaceutical industry.
For instance, according to an article published by Life Sciences Review, as drug discovery and development grow more complex, pharmaceutical companies are increasingly relying on Life Sciences Business Process Outsourcing (BPO) to enhance efficiency, reduce expenses, and speed up research efforts.
Pharmaceutical companies’ segment is estimated to contribute the highest market share of 40.62% in 2025. Drug makers continue to outsource non-core functions in order to streamline internal operations and concentrate on high-value strategic activities. Their outsourcing requirements extend across the entire value chain from discovery and pre-clinical testing to commercialization support. Clinical development presents the biggest opportunity due to scale and complexity of trials. Pharma companies partner with CROs to execute global multi-site studies and leverage specialized technical expertise. Manufacturing and quality testing is also increasingly handled by contract manufacturing organizations (CMO) to gain capacity and flexibility. On the commercial front, contract sales organizations (CSO) are engaged for promotional activities in select therapeutic areas and geographical markets.
In March 2025, Shilpa Medicare, based in Raichur, launched a new full-service hybrid CDMO, supporting both small and large molecule development, including peptides, with a focus on oncology. The hybrid model offers end-to-end discovery, clinical, and commercial services, along with ready-to-license novel formulations for B2B partners. This dual approach allows pharma companies to benefit from Shilpa’s oncology expertise while minimizing development risks and timelines.
To learn more about this report, Download Free Sample
The Life Sciences BPO industry in North America is undergoing transformation as providers actively embrace digital technologies and integrated service models. Leading firms are enhancing clinical data management, pharmacovigilance, and regulatory processes by incorporating AI, automation, and real-world evidence analytics. The region’s strong pharmaceutical and biotech presence, coupled with complex regulations and rising cost pressures, is driving companies to outsource non-core functions.
Asia Pacific leads the global life sciences business process outsourcing industry, with pharmaceutical services generating most of the revenue. Medical device outsourcing is growing rapidly, especially in China and India, due to cost advantages and a skilled workforce. BPO providers are driving decentralized trials and remote patient monitoring by leveraging telehealth and digital platforms to improve site accessibility, data quality, and patient retention.
Canadian CROs are actively integrating artificial intelligence, machine learning, and real-world evidence analytics into decentralized and hybrid clinical trials. They use mobile wearables, e-consent, and AI-driven recruitment to accelerate timelines, expand patient access across regions, and uphold the integrity of key oncology studies. To address payer demands and simplify vendor management, Canadian BPO providers now offer unified contracts that bundle clinical, commercial, and digital services, linking compensation to patient outcomes, cost efficiency, and milestone achievements.
Life sciences companies are shifting their outsourcing strategies by choosing nearshore hubs in Eastern and Central Europe over distant offshore locations. Countries such as Poland, Hungary, and Czechia offer cost-effective services, skilled talent, and regulatory alignment with the EMA. At the same time, BPO providers are leading efforts to digitize clinical trial management and regulatory documentation. They employ AI-powered eCTD publishing, e-signature systems, and remote monitoring tools to boost accuracy, speed up submissions, and ensure compliance with evolving EU Clinical Trial Regulation (CTR) standards.
To learn more about this report, Download Free Sample
In January 2025, IQVIA, a clinical research, commercial analytics, and healthcare intelligence company , announced a strategic collaboration with NVIDIA to unlock the potential of AI in healthcare and life sciences.
In December 2024, Infosys announced a ₹8.3 crore (approximately USD 1 million) investment in healthcare and life sciences startup 4baseCare through the Infosys Innovation Fund.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 462.83 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 13.4% | 2032 Value Projection: | USD 1,116.81 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
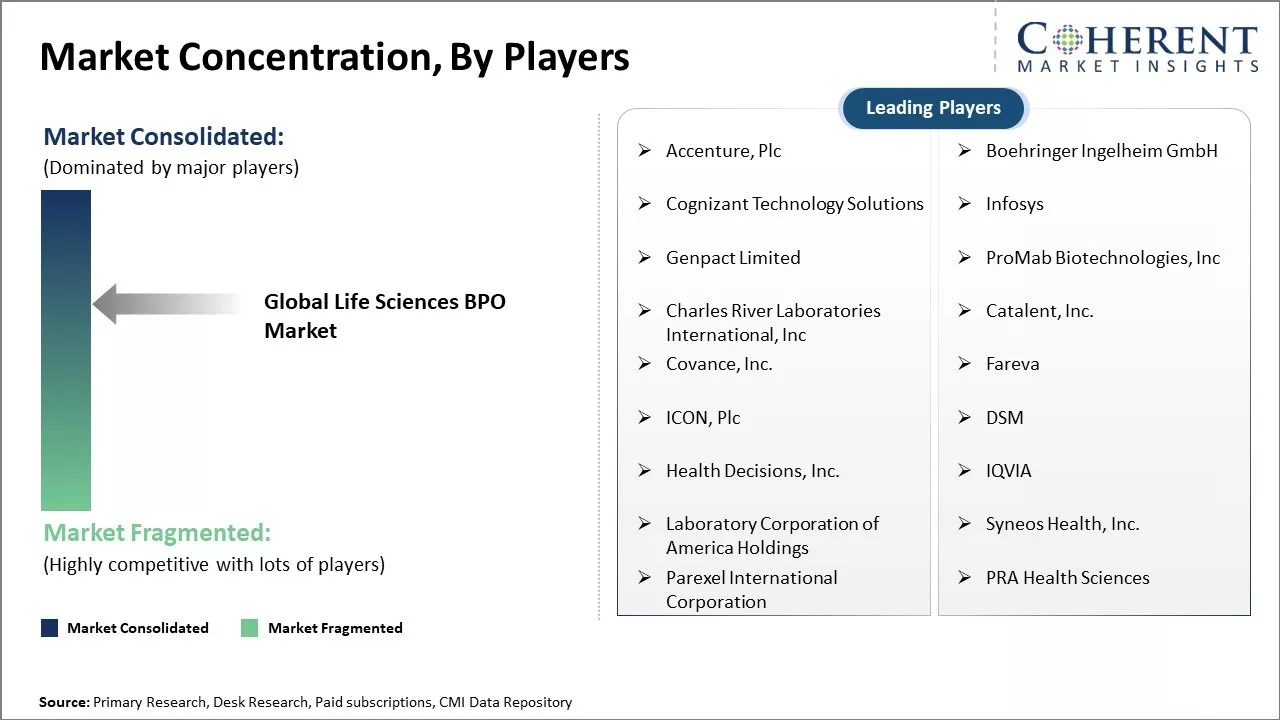
Accenture, Plc, Boehringer Ingelheim GmbH, Cognizant Technology Solutions, Infosys, Genpact Limited, ProMab Biotechnologies, Inc, Charles River Laboratories International, Inc, Catalent, Inc., Covance, Inc., Fareva, ICON, Plc, DSM, Health Decisions, Inc., IQVIA, Laboratory Corporation of America Holdings, Syneos Health, Inc., Parexel International Corporation, PRA Health Sciences |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
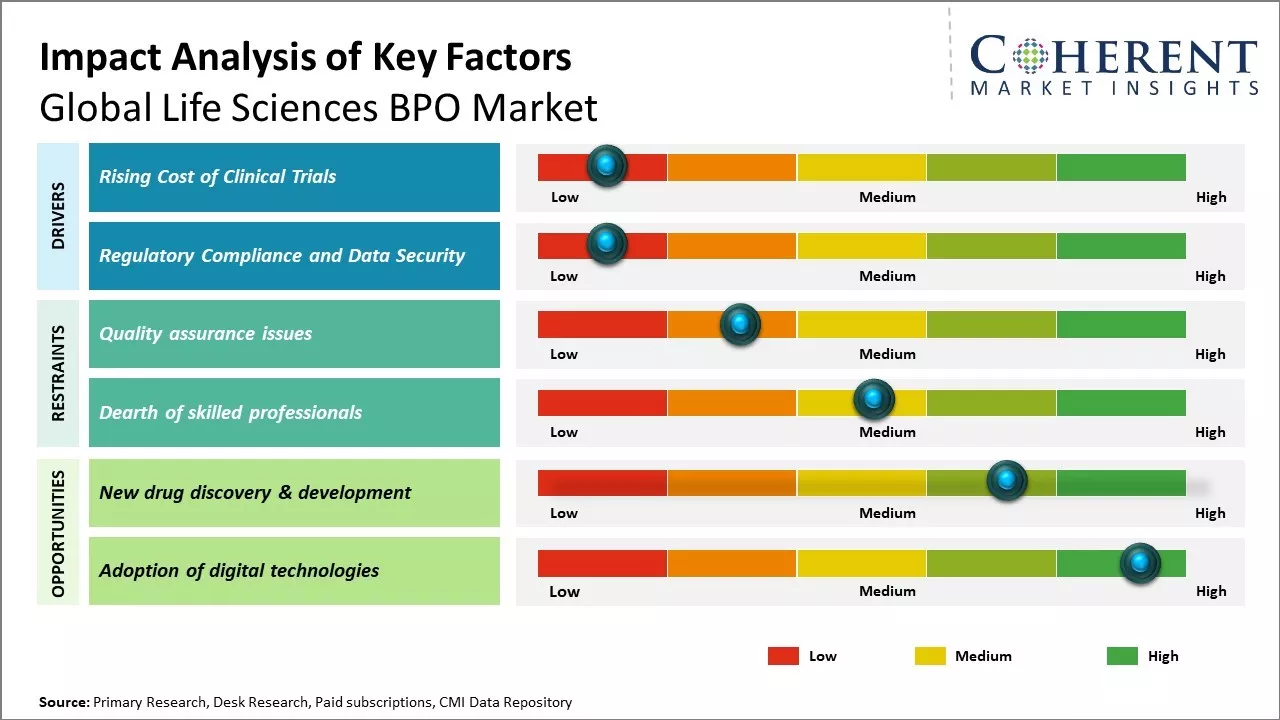
With rising R&D spending in the pharmaceutical and biotech industry, the costs associated with clinical drug trials have also exponentially increased. Clinical trials have become lengthy and complex procedures involving thousands of patients enrolled across hundreds of sites worldwide. The management of these large-scale clinical operations requires specialized skills and expertise that many biopharma companies lack in-house. Outsourcing non-core functions like patient recruitment and retention, site management, laboratory sample testing, and clinical data management to specialist BPO providers has become increasingly attractive as it allows biopharma companies to focus on their core drug development work.
Regulators across the world are placing greater scrutiny on clinical trial conduct and are imposing stricter rules for data privacy, integrity, and security. From the FDA to the EMA to Japan's PMDA, regulators are ramping up GXP inspections and enforcing heavy penalties for non-compliance issues. The regulatory landscape in life sciences has become highly complex with a plethora of global guidelines and directives that sponsors must adhere. Ensuring full compliance and maintaining customizable systems for safety reporting, document control, and auditing is challenging for most biopharma companies. By leveraging BPO providers that have extensive regulatory expertise and technology-enabled platforms, sponsors can address compliance in a more efficient, low risk manner.
The global shift toward decentralized and hybrid clinical trial models offers BPO providers a major opportunity. Sponsors are increasingly outsourcing trial components like remote monitoring, e-consent, and virtual patient engagement. BPOs that invest in telehealth infrastructure, wearable data integration, and regulatory-compliant digital platforms can capture significant value. As sponsors prioritize faster enrollment and patient-centric approaches, demand for end-to-end support in managing decentralized protocols will accelerate across both developed and emerging markets.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients