Kyphoplasty and Percutaneous Discectomy Procedure Market is estimated to be valued at USD 2,455.2 Mn in 2025 and is expected to reach USD 3,619.2 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 5.7% from 2025 to 2032. Utilizing kyphoplasty, a particular cement is injected into the vertebrae, and an additional step is taken to make room for the procedure with a balloon-like device (balloon vertebroplasty). Kyphoplasty can increase the height of a damaged vertebra and may also be pain-relieving.
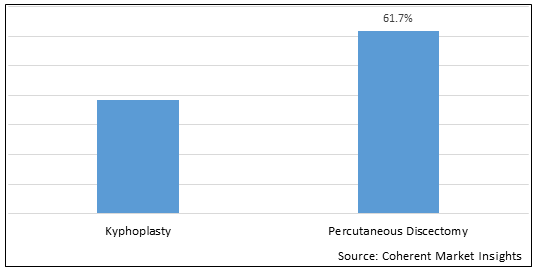
Figure 1.Global Kyphoplasty and Percutaneous Discectomy Procedure Market Share (%), by Procedure, 2025
To learn more about this report, Download Free Sample
Global Kyphoplasty and Percutaneous Discectomy Procedure Market- Drivers
Increasing prevalence of Vertebral Compression Fractures (VCF), osteoporosis, and other spinal disorders is expected to drive market growth over the forecast period. For instance, according to the American Association of Neurological Surgeons, VCFs are the most common fracture in patients with osteoporosis, that affect about 750,000 people annually in the U.S. VCFs affect an estimated 25% of all postmenopausal women in the U.S.
The increasing demand for minimally invasive surgical procedures in orthopedics and thereby improvements in reimbursement policies is expected to further drive the market growth. For instance, in September 2020, the SpineJack implantable fracture reduction system of Stryker, a multinational medical technologies company, received the U.S. Centers for Medicare & Medicaid Services (CMS) New Technology Add-on Payment (NTAP) as part of the 2021 inpatient prospective payment system. The SpineJack System is an implanted fracture reduction system, intended to reduce vertebral compression fractures. After the SpineJack implant is inserted, it is expanded, and Vertaplex HV bone cement is injected at a low pressure to stabilize the restored vertebral body.
Kyphoplasty and Percutaneous Discsectomy Procedure Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2,455.2 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.7% | 2032 Value Projection: | USD 3,619.2 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Stryker, Medtronic Inc., BPB Medica – Biopsybell, Smith & Nephew, MicroPort Scientific Corporation, Johnson & Johnson, Halma plc, Becton, Dickinson and Company , Merit Medical Systems, Joimax GmbH, G21 S.r.l., Joline GmbH & Co. KG, Seawon MediTech |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
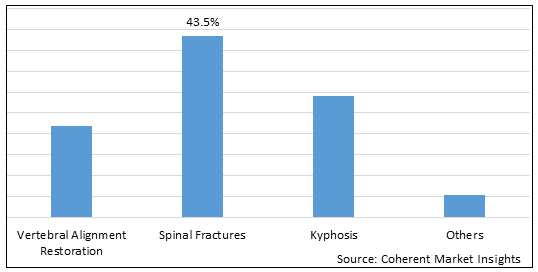
Figure 2. Global Kyphoplasty and Percutaneous Discectomy Procedure Market Share (%), by Application, 2025
To learn more about this report, Download Free Sample
Global Kyphoplasty and Percutaneous Discectomy Procedure Market– Impact of Coronavirus (COVID-19) Pandemic
Lockdown or shut down due to covid 19 was imposed in many countries globally, which had a negative impact on the economy of the private healthcare sector globally. This lockdown resulted in the closure of industrial establishments, except for the manufacturing of essential commodities, and disruption in the supply chain of products. Thus, the COVID-19 pandemic has affected the economy in three main ways: by directly affecting the production and demand o by creating disruptions in distribution channels o through its financial impact on firms and financial markets. The COVID-19 outbreak is anticipated to have a negative impact on the global kyphoplasty and percutaneous discectomy procedure market. Several clinics and hospitals all around the world undertook restructuring to increase hospital capacity for patients with COVID-19 diagnoses. The high rise in COVID-19 cases resulted in a potential backlog in non-essential procedures. For instance, as per a study published in May 2020, in Asian Spine Journal, spine surgical procedures decreased by approximately 50% in the first month of covid wave in France as compared to the same period in 2019.
Global Kyphoplasty and Percutaneous Discectomy Procedure Market: Key Developments
In September 2021, Spinal Elements, a spine technology company, announced the full commercial launch of the Luna XD multiexpandable lumbar interbody fusion device and Orbit articulating discectomy systems. Luna XD and Orbit have been integrated into Spinal Elements as the newest technologies in its Minimally Invasive Spine (MIS) Ultra platform of products and procedural solutions.
In January, Merit Medical Systems, a manufacturer and marketer of proprietary disposable medical devices, announced the official market launch of Arcadia Steerable and Straight Balloons for vertebral augmentation. Arcadia balloons are designed to help physicians achieve controlled, precise cavity creation during unipedicular or bipedicular vertebral augmentation and kyphoplasty procedures
Global Kyphoplasty and Percutaneous Discectomy Procedure Market: Restraint
The high cost of minimally invasive procedures is expected to hamper the market growth. The price of spinal surgeries is very high, which can hamper the market growth. For instance, according to New Choice Health, Inc., a free, consumer-focused healthcare marketplace, the national average cost of vertebral augmentation surgery such as vertebroplasty and kyphoplasty is US$ 36,880 in the U.S. According to the same source, the average price range for kyphoplasty is US$ 11,300 to US$ 31,000 in the U.S.
Global Kyphoplasty and Percutaneous Discectomy Procedure Market- Key Players
Major players operating in the global kyphoplasty and percutaneous discectomy procedure market include Stryker, Medtronic Inc., BPB Medica – Biopsybell, Smith & Nephew, MicroPort Scientific Corporation, Johnson & Johnson, Halma plc, Becton, Dickinson and Company, Merit Medical Systems, Joimax GmbH, G21 S.r.l., Joline GmbH & Co. KG, and Seawon MediTech
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients