Global intravenous solutions market is estimated to be valued at USD 11.05 Bn in 2025 and is expected to exhibit a CAGR of 6.3% during the forecast period (2025-2032). Intravenous solutions (IV) are administered in patients to provide sugar, salt and water directly into venous circulation. IV solutions and electrolytes are used for fluid resuscitation, routine maintenance, replacement, and redistribution. Nowadays, intravenous drugs, nutrition and mixed solutions have become an integral part of modern therapy. The intravenous route of administration is commonly used for rehydration or to provide nutrients for those who cannot, or will not, due to reduced mental states or otherwise, consume food or water by mouth. It may also be used to administer medications or other medical therapy such as blood products or electrolytes to correct electrolyte imbalances.
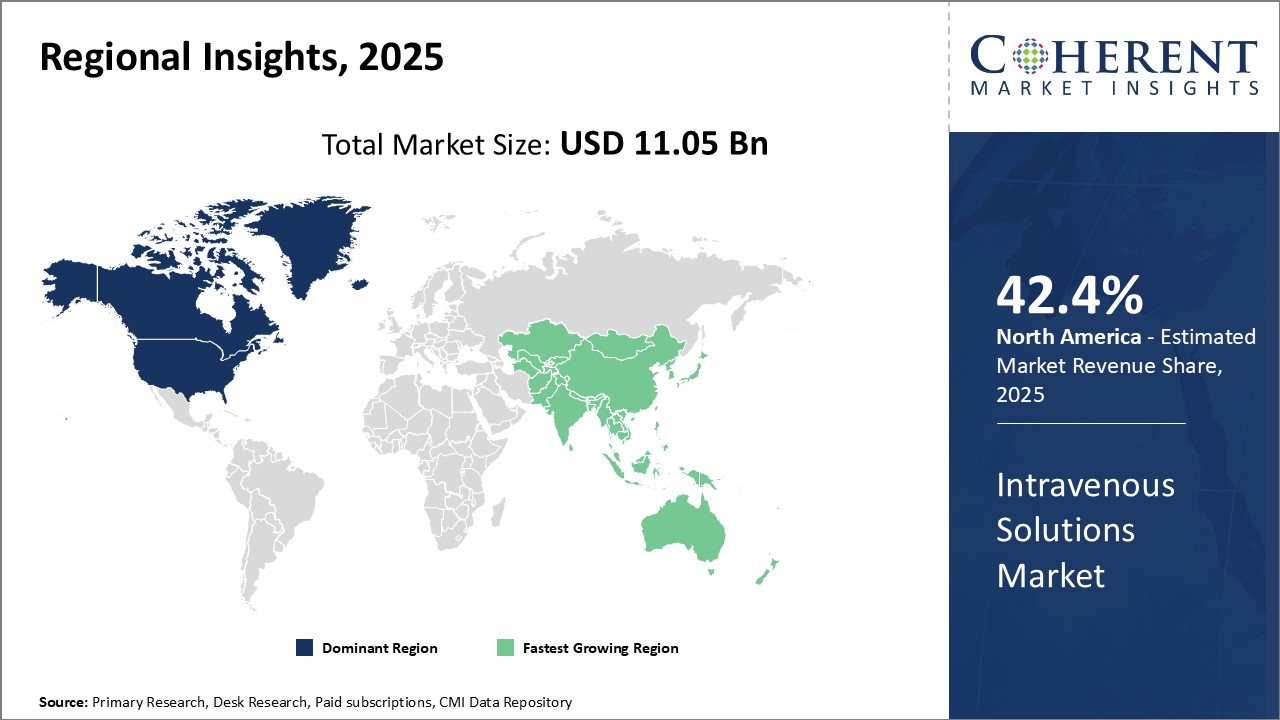
Figure 1. Global Intravenous Solutions Market Value (US$ Bn), by Region, 2025
To learn more about this report, Download Free Sample
Increasing launches and regulatory approvals for intravenous solution injection is expected to drive growth of the global intravenous solution market
Market players are involved in launching new intravenous injection solutions which is expected to boost the growth of global intravenous solutions market over the forecast period. For instance, in September 2021, Baxter International Inc., a global sterile medication production company, announced the U.S. Food and Drug Administration (FDA) approval and commercial launch of premix Norepinephrine Bitartrate in 5% Dextrose Injection (norepinephrine). Norepinephrine is indicated to raise blood pressure in adult patients with severe, acute hypotension (low blood pressure). Baxter’s formulation of norepinephrine is the first and only manufacturer-prepared ready-to-use formulation and is available in 4 mg/250 mL (16 mcg/mL) and 8 mg/250 mL (32 mcg/mL) strengths.
Intravenous Solutions Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 11.05 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.3% | 2032 Value Projection: | USD 16.95 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Baxter International Inc., ICU Medical. Inc, B. Braun Melsungen Ag, Grifols, S.A., Fresenius Kabi USA, LLC, Vifor Pharma Management Ltd, JW Life Science, Amanta Healthcare, Axa Parenterals Ltd, and Salius Pharma Private Limited, Pfizer, Inc, Otsuka Pharmaceutical Co., Ltd, Ajinomoto Co., Inc., B. Braun Melsungen AG, Soxa Formulations & Research Pvt.Ltd, Sichuan Kelun Pharmaceutical Co Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
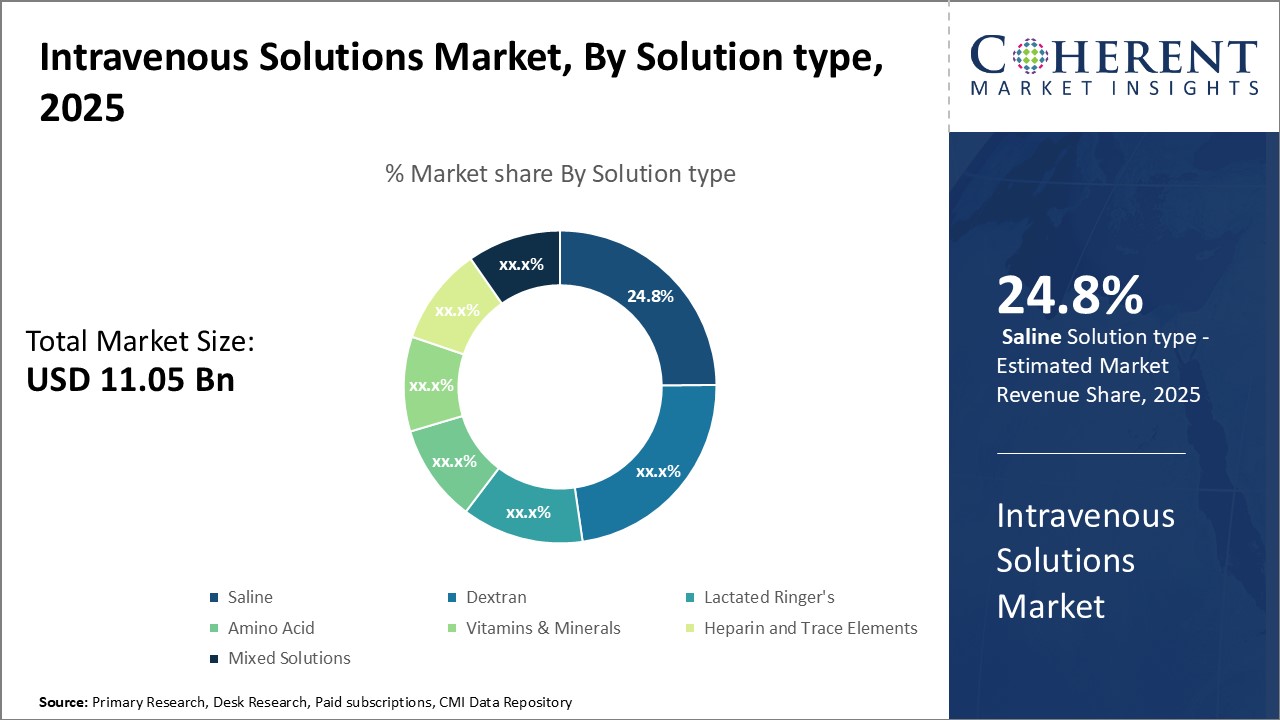
Figure 2. Global Intravenous Solutions Market Share, By Solution, 2025
To learn more about this report, Download Free Sample
Increasing launches of parenteral (intravenous) nutrition (PN) products by key market players is attributing to the highest share of North America market in the global intravenous solutions market.
Increasing launches of parenteral (intravenous) nutrition (PN) products by key players in market for developing global intravenous solutions is expected to drive market growth over the forecast period. For instance, in June 2019, Eurolife Healthcare, a manufacturer and distributor of specialty infusion & pharmaceuticals, launched two intravenous IV products: Discport and Lifeport in the India drug market. The product is considered as smart intravenous infusion, which encompasses the latest technology that reduces the chances of any infection.
Global Intravenous Solutions Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization had declared it a public health emergency on January 30, 2020.
The sudden outbreak of COVID-19 has bought the world to a standstill. The demand for IV solutions has increased due to the fast spread of coronavirus illness (COVID-19), which will allow patients in intensive care units to receive vital nutrients (ICU). In addition to this, a significant growth-inducing element is the rise in diabetes, cancer, gastrointestinal problems, and neurological illnesses. Additionally, IV solution therapy's superior efficacy and quicker response time are fostering market expansion. It is employed to treat chronic dehydration, a disease that can lead to weariness, memory loss, irritability, and other health issues. For instance, in February 2022, the World Health Organization published a report which stated that nearly 10 million deaths in 2020, or nearly one in six deaths globally due to cancer. Cancer-causing infections, such as human papillomavirus (HPV) and hepatitis, are responsible for approximately 30% of cancer cases in low- and lower-middle-income countries like India and Bangladesh.
Thus, impact of the Coronavirus (COVID-19) pandemic has driven growth of the global intravenous solutions market during the forecast period.
Recent Developments
Intravenous Solutions Market Restraints
Growth of the global intravenous solution market is expected to be hampered over the forecast period, owing to increasing FDA recalls of IV products. For instance, in August 2018, the U.S. FDA recalled Becton Dickinson & Company’s NEXIVA Closed IV Catheter System Dual Port 18GA 1.25 IN (BD Nexiva catheter) due to failure in the needle tip shield/safety mechanism.
Key Players
Key players operating in the global intravenous solutions market include Baxter International Inc., ICU Medical. Inc, B. Braun Melsungen Ag, Grifols, S.A., Fresenius Kabi USA, LLC, Vifor Pharma Management Ltd, JW Life Science, Amanta Healthcare, Axa Parenterals Ltd, and Salius Pharma Private Limited, Pfizer, Inc, Otsuka Pharmaceutical Co., Ltd, Ajinomoto Co., Inc., B. Braun Melsungen AG, Soxa Formulations & Research Pvt.Ltd, Sichuan Kelun Pharmaceutical Co Ltd.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients