Intravascular Ultrasound Imaging System Market Size and Forecast – 2025 – 2032
The Global Intravascular Ultrasound Imaging System Market size is estimated to be valued at USD 1.2 billion in 2025 and is expected to reach USD 2.3 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 10.5% from 2025 to 2032.
Global Intravascular Ultrasound Imaging System Market Overview
Intravascular ultrasound (IVUS) imaging systems are catheter-based diagnostic devices that use high-frequency sound waves to visualize the interior of blood vessels. These systems consist of miniaturized transducers mounted on catheters, real-time image processors, and display consoles. IVUS allows clinicians to assess plaque morphology, vessel diameter, and stent placement with exceptional accuracy. Modern devices feature high-resolution digital imaging, automated pullback mechanisms, and integration with optical coherence tomography (OCT) for multimodal imaging. The latest designs emphasize improved catheter flexibility, smaller profiles, and AI-enabled image interpretation.
Key Takeaways
The catheter-based IVUS system remains the most significant contributor to market revenue, with strong adoption in coronary artery disease applications demonstrating sustained growth.
In terms of end-users, hospitals dominate market share due to their comprehensive cardiovascular care infrastructure, whereas ambulatory surgical centers are one of the fastest-growing adopters owing to increased minimally invasive procedures.
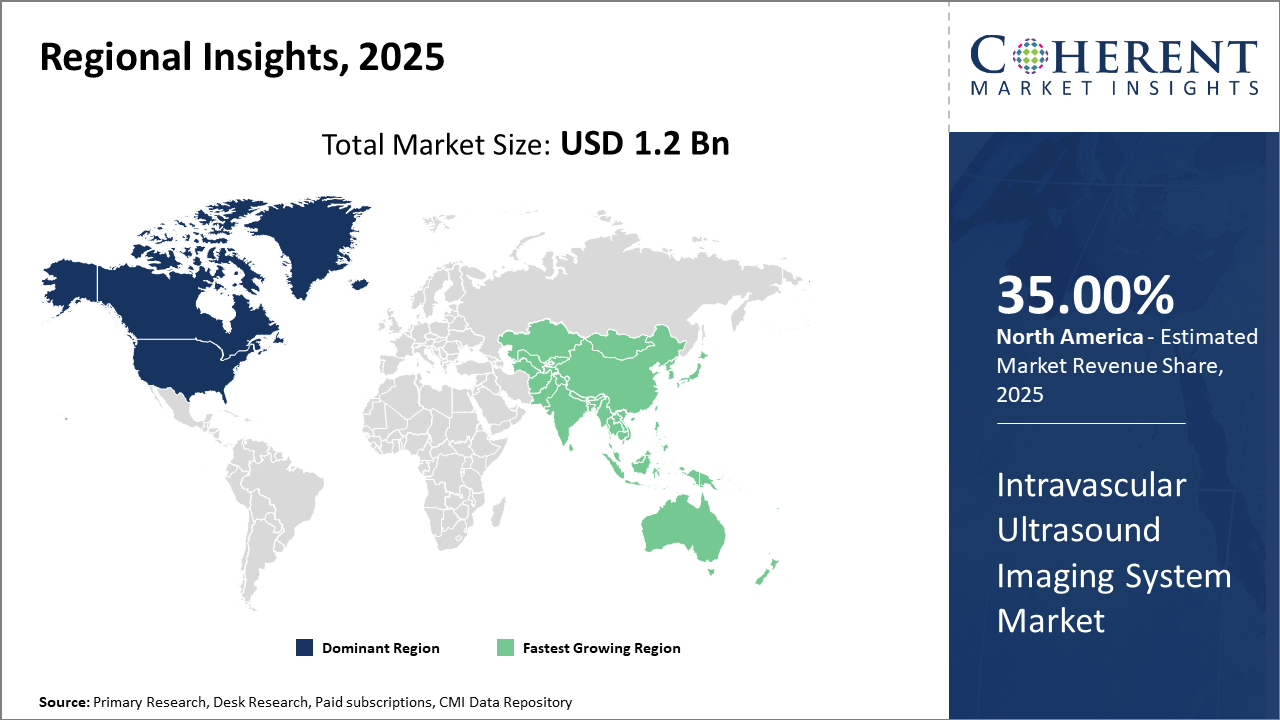
Regionally, North America leads the market share with over 35% of the industry revenue, supported by advanced healthcare infrastructure and high procedural volumes.
Meanwhile, Asia Pacific exhibits the highest CAGR, exceeding 12% due to rising cardiovascular disease prevalence and growing healthcare investments in emerging markets such as India and China.
Intravascular Ultrasound Imaging System Market Segmentation Analysis
To learn more about this report, Download Free Sample
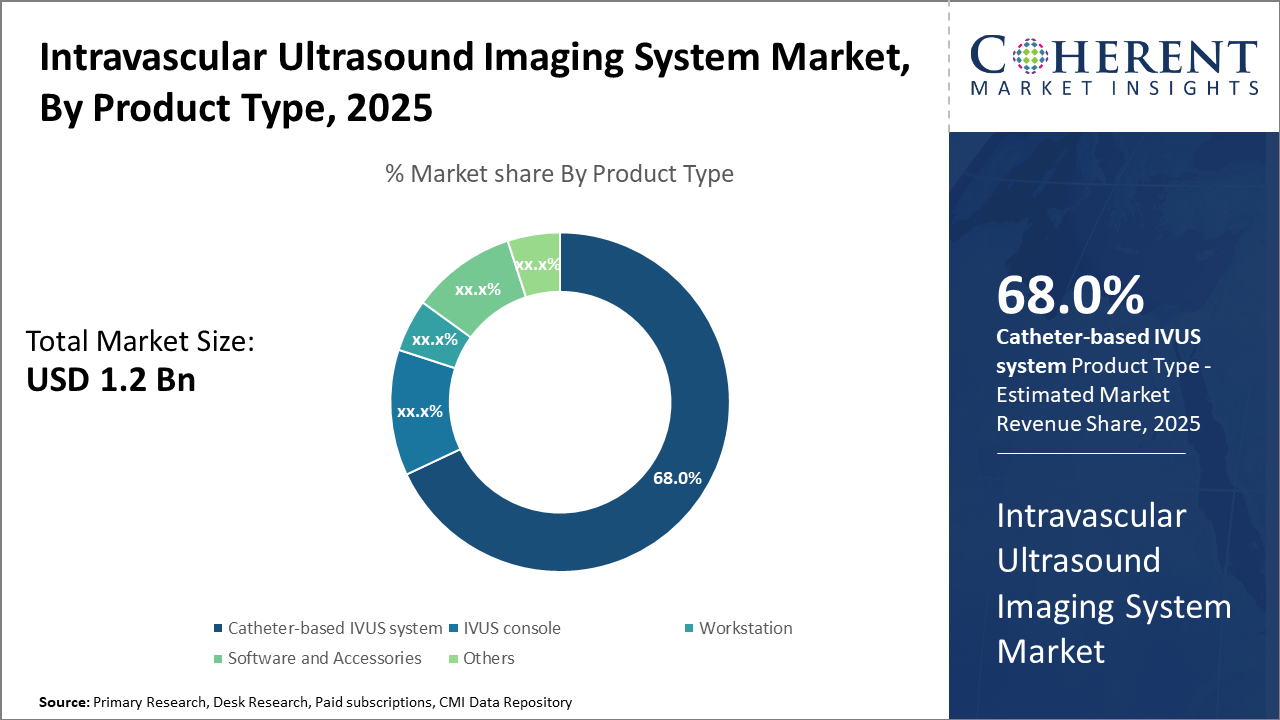
Intravascular Ultrasound Imaging System Market Insights, By Product Type
Catheter-based IVUS systems dominate the market share with 68%, largely due to their critical role in delivering high-resolution vascular images during interventions. This subsegment’s growth is propelled by continuous miniaturization efforts and the inclusion of disposable catheter designs that reduce infection risks. The fastest-growing segment is the software and accessories segment, accelerated by the integration of AI-driven analytical tools that enhance image interpretation accuracy and procedure efficiency. IVUS consoles remain a vital but mature subsegment, offering control and processing capabilities, whereas workstations cater to analysis and reporting needs but grow comparatively more slowly.
Intravascular Ultrasound Imaging System Market Insights, By Application
Coronary artery disease leads the segment, driven by the sheer volume of percutaneous coronary interventions globally. Advancements in IVUS-guided stent placements are leading to higher procedural success rates and expanded use across specialized interventions. Peripheral artery disease is the fastest-growing application segment, fueled by rising awareness and adoption in treating lower limb ischemia with IVUS guidance, improving diagnostic precision. Chronic total occlusions account for a smaller but stable share, benefiting from growing off-label usage of IVUS in complex lesion imaging.
Intravascular Ultrasound Imaging System Market Insights, By End-User
Hospitals dominate the market, attributed to their integrated cardiovascular services and large patient inflow, making them primary adopters of IVUS systems. Cardiac centers, specializing in interventional cardiology and electrophysiology, are pivotal players with growing adoption supported by evidence linking IVUS usage to improved clinical outcomes. Ambulatory surgical centers represent the fastest-growing subsegment, driven by an increase in minimally invasive procedures performed in outpatient settings and cost-efficiency initiatives.
Intravascular Ultrasound Imaging System Market Trends
The intravascular ultrasound imaging system market is witnessing a technological convergence trend, notably the integration of AI algorithms in image processing, which enhances the accuracy and speed of vascular assessments.
For instance, a pivotal study in 2025 demonstrated that AI-assisted IVUS reduced operator dependency errors by nearly 12%, accelerating diagnostic workflows in top-tier cardiovascular centers.
Furthermore, the expansion of hybrid imaging systems combining IVUS with complementary modalities like OCT is streamlining lesion assessment, thereby improving patient stratification during percutaneous interventions.
This technology fusion is poised to redefine procedural standards and expands the market scope.
Intravascular Ultrasound Imaging System Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Intravascular Ultrasound Imaging System Market Analysis and Trends
In North America, the dominance in the Intravascular Ultrasound Imaging System market is powered primarily by the U.S., which benefits from advanced research infrastructure, high procedural volumes in coronary and peripheral artery disease, and extensive government and private funding. The presence of major players such as Abbott Laboratories and Boston Scientific, contributing with cutting-edge IVUS platforms, underpins this leadership. Supportive reimbursement policies and a growing elderly population with cardiovascular ailments further sustain market revenue.
Asia Pacific Intravascular Ultrasound Imaging System Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with a CAGR exceeding 12% due to increasing investments in healthcare, especially in urban centers of India and China. Expansion of hospital infrastructure, coupled with rising awareness and screening programs for cardiovascular diseases, fuels demand. Local manufacturers are scaling capabilities, reducing costs, and increasing accessibility. Additionally, foreign direct investments and regulatory reforms have lowered barriers, facilitating rapid market penetration.
Intravascular Ultrasound Imaging System Market Outlook for Key Countries
USA Intravascular Ultrasound Imaging System Market Analysis and Trends
The USA represents the lion’s share of the North American Intravascular Ultrasound Imaging System market, accounting for over 90% of regional revenue in 2025. Driving factors include a high prevalence of coronary artery disease, estimated at nearly 18.2 million affected adults, and the growing adoption of IVUS-guided interventions improving clinical outcomes. Industry leaders like Philips Healthcare and Abbott Laboratories have launched next-gen IVUS systems featuring AI-based image analysis here, resulting in their market share increment by approximately 10% in 2024. Favorable regulatory approvals and reimbursement policies further bolster the country’s market growth.
India Intravascular Ultrasound Imaging System Market Analysis and Trends
India's Intravascular Ultrasound Imaging System market is undergoing rapid transformation owing to escalating ischemic heart disease cases, with over 2.7 million annual cardiovascular deaths reported in 2024. The government's push to upgrade healthcare infrastructure and support for indigenous manufacturing has fostered access to cost-effective IVUS technologies. Key market players focus on joint ventures and product launches tailored for the local market’s pricing sensitivity. Widening healthcare insurance and increasing awareness among cardiologists are driving adoption, with market revenue growth rates outpacing many global counterparts.
Analyst Opinion
Key demand-side insight highlights a surge in procedural volumes involving intravascular ultrasound (IVUS). Notably, cardiovascular procedures using IVUS-guided techniques grew by approximately 15% in the US alone during 2024, driven by physician preference for improving stent placement accuracy, contributing directly to market size expansion.
Supply-side analysis reveals that enhanced production capacity of IVUS catheters and systems, with over 20 million catheters produced globally in 2024, has reduced lead times and improved market accessibility, fostering increased device adoption. In addition, pricing strategies have evolved to offer modular devices with advanced imaging features at competitive price points, aiding penetration in emerging markets.
From a technological micro-indicator perspective, integration of artificial intelligence (AI) in IVUS image analysis has reduced diagnostic errors by an estimated 12% in pilot clinical settings in 2025, enabling better treatment planning and significantly influencing market growth trajectories.
An import-export trend shows rising equipment imports in Asia Pacific, particularly for high-end systems, supplemented by local manufacturing activities in countries such as India and China. This market dynamic has contributed to regional market growth, accounting for nearly 28% of total Asia Pacific market revenue in 2025.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: |
USD 1.2 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.5% | 2032 Value Projection: |
USD 2.3 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Abbott Laboratories, Boston Scientific Corporation, Philips Healthcare, Siemens Healthineers, Terumo Corporation, Canon Medical Systems Corporation, GE Healthcare, Biosensors International Group, Volcano Corporation, Siemens AG, Samsung Medison, Medtronic plc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Intravascular Ultrasound Imaging System Market Growth Factors
The rising prevalence of cardiovascular diseases globally, such as coronary artery disease affecting over 20 million individuals annually, is fuelling demand for advanced diagnostic tools like intravascular ultrasound imaging systems. Technological advancements, including miniaturized catheters and enhanced imaging resolution, have increased adoption in complex vascular procedures. Moreover, increasing clinical evidence supporting IVUS-guided interventions correlates with improved patient outcomes, thus encouraging hospitals to invest in these systems. The growing healthcare infrastructure in emerging economies, combined with favorable reimbursement policies, further propels market expansion.
Intravascular Ultrasound Imaging System Market Development
In November 2024, Boston Scientific Corporation launched AVVIGO, an AI-enabled multi-modality intravascular ultrasound (IVUS) and fractional flow reserve (FFR) system designed to enhance precision in coronary imaging and functional assessment. The system leverages artificial intelligence to deliver advanced plaque characterization, optimize stent placement, and support clinicians in achieving improved accuracy and outcomes in interventional cardiology procedures.
In September 2024, Boston Scientific Corporation released the next-generation version of its iLab System Software, offering a comprehensive 360-degree view of vascular structures for superior diagnostic and treatment planning. The upgraded platform integrates enhanced imaging analytics and intuitive visualization tools, enabling cardiologists to make more precise assessments and deliver better patient outcomes during complex cardiovascular interventions.
Key Players
Leading Companies of the Market
Abbott Laboratories
Philips Healthcare
Siemens Healthineers
Terumo Corporation
Canon Medical Systems Corporation
GE Healthcare
Biosensors International Group
Volcano Corporation
Siemens AG
Samsung Medison
Medtronic plc
Several leading market players have adopted strategic collaborations and product innovation approaches to sustain competitive advantage. For example, Abbott Laboratories launched an AI-powered IVUS system in 2024, leading to a 20% increase in market share in North America. Boston Scientific’s expansion into emerging markets through partnerships in India and Southeast Asia has demonstrated a 14% year-over-year revenue increase. Philips Healthcare’s focus on seamless integration of IVUS with other imaging modalities has enhanced clinical workflow efficiency, reinforcing its strategic position globally.
Intravascular Ultrasound Imaging System Market Future Outlook
Next-generation IVUS systems will feature AI-assisted imaging analysis, automated vessel characterization, and 3D reconstruction capabilities. The convergence of IVUS with optical coherence tomography (OCT) and near-infrared spectroscopy will provide comprehensive vascular insight in a single procedure. Catheters with higher flexibility and smaller diameters will enable safer, faster interventions. Wireless and real-time cloud-linked IVUS platforms are expected to support remote procedure guidance and data sharing. As precision cardiovascular interventions expand, IVUS will continue to evolve as a critical imaging modality for interventional cardiology.
Intravascular Ultrasound Imaging System Market Historical Analysis
Intravascular ultrasound (IVUS) imaging systems emerged in the late 1980s as a revolutionary tool for real-time visualization of vascular structures. Early analog systems provided cross-sectional views of arteries, helping clinicians evaluate plaque burden and vessel dimensions. The digital transformation of IVUS in the 2000s significantly improved image resolution and quantitative accuracy. Integration with coronary interventions, especially stent placement, established IVUS as a key adjunct to angiography. Progressive miniaturization of catheters and enhanced image processing cemented its clinical utility.
Sources
Primary Research Interviews:
Interventional Cardiologists
Medical Imaging Engineers
Clinical Device Specialists
Biomedical Technicians
Databases:
FDA Device Database
GlobalData Cardiovascular Devices Reports
WHO Noncommunicable Diseases Data
Magazines:
Cardiovascular Business
MedTech Insight
Diagnostic and Interventional Cardiology
Medical Design Briefs
Journals:
Journal of the American College of Cardiology
Catheterization and Cardiovascular Interventions
Newspapers:
The Wall Street Journal (Health)
The Guardian (Science)
The New York Times (Medicine)
The Hindu (Health)
Associations:
American College of Cardiology (ACC)
European Society of Cardiology (ESC)
Society for Cardiovascular Angiography and Interventions (SCAI)
World Heart Federation (WHF)
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients