An interventional intracranial aneurysm is an unusual central dilation of an artery in the brain that results from a weakening of the inner muscular layer of a blood vessel wall. An intracranial aneurysm can leak, causing bleeding into the brain. Most often, a ruptured brain aneurysm occurs in the space between the brain and the thin tissues covering the brain.
Intracranial aneurysm diagnostic tests include computerized tomography, cerebrospinal fluid test, magnetic resonance imaging, cerebral angiogram. Intracranial aneurysm can be treated by surgery such as brain aneurysm surgery which include surgical clipping, a procedure to close off an aneurysm, as well as endovascular treatment.
Other treatments for ruptured aneurysms includes
Global Interventional Intracranial Aneurysm Devices Market - Impact of the Coronavirus (COVID-19) Pandemic
The COVID-19 pandemic is expected to hamper the growth of the global interventional intracranial aneurysm devices market. Lockdown was imposed in many countries due to the COVID-19 which had a negative impact on the economy of the private healthcare sector globally. The lockdown resulted in the closure of industrial establishments, except the manufacturing of essential commodities. There was a disruption in the supply chain of products. Consequently, the COVID-19 pandemic has affected the economy by directly affecting production and demand, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets.
The COVID-19 pandemic had a negative economic impact on the global interventional intracranial aneurysm devices market, owing to the decreased number of surgeries during the COVID-19 pandemic. For instance, according to an article published by the National Center for Biotechnology Information, a set of databases providing access to biomedical and genomic information, in December 2020, the weekly rate of surgical procedures did not decrease significantly during week 1 of the COVID period (March 11, 2020–March 31, 2020) when compared to the equivalent period in 2019. However, it decreased by 78 percent by week 2 and 83 percent by week 3 as compared to the equivalent period in 2019. This study was carried out in Ontario, Canada by utilizing health administrative data sets of all hospital-based scheduled and urgent surgical procedures.
The global interventional intracranial aneurysm devices market is estimated to be valued at US$ 3,670.34 Mn in 2022 and is expected to exhibit a CAGR of 7.7% over the forecast period (2022-2030)
Figure 1: Global Interventional Intracranial Aneurysm Devices Market Share, (%), Analysis, By Intracranial Aneurysm Type, 2022
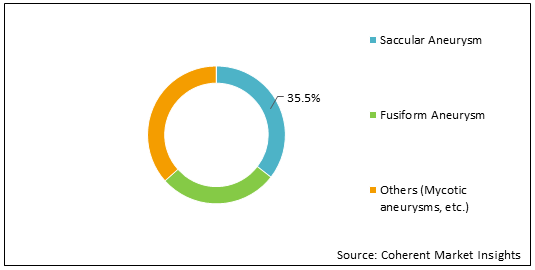
To learn more about this report, Download Free Sample
Market players are adopting inorganic growth strategies such as partnerships, to strengthen its position in the global interventional intracranial aneurysm market. This is expected to drive growth of the interventional intracranial aneurysm devices market over the forecast period.
Market players are adopting inorganic growth strategies such as partnerships, this is expected to drive growth of the global interventional intracranial aneurysm devices market over the forecast period. For instance, in June 2022, Stryker, a worldwide medical technology company, announced a partnership with Carmeda AB, a Sweden-based medical device company, to combine Stryker's proven flow diverter technology with Carmeda's active heparin coating for the treatment of brain aneurysms.
Interventional Intracranial Aneurysm Devices Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 3,670.34 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 7.7% | 2030 Value Projection: | US$ 6,632.92 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
MicroPort Scientific Corporation, B. Braun SE, Stryker, MicroVention Inc., Johnson & Johnson Private Limited, Terumo Corporation, Penumbra, Inc., KANEKA CORPORATION, Perflow Medical Ltd, phenox GmbH, Evasc, ASAHI INTECC CO., LTD., Acandis GmbH, Imperative Care, Medtronic |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Research and academic institutes are engaged in developing technologically advancement products and surgical procedures for the treatment of brain or intracranial aneurysm, which is expected to drive the global interventional intracranial aneurysm devices market growth over the forecast period.
Research and academic institutes are engaged in developing technologically advancement products and surgical procedures for the treatment of brain or intracranial aneurysm, which is expected to drive the global interventional intracranial aneurysm devices market growth over the forecast period. For instance, in May 2021, a study team from Pohang University of Science and Technology (POSTECH), a university in South Korea, had developed a novel method of filling of blood in aneurysms that can deactivate the rupturing of blood vessels. The interdisciplinary research team at POSTECH had created a novel structurally stable, biocompatible embolization material that does not degrade in the human body to address the drawbacks of coil embolization. A novel idea for cerebral aneurysm therapy (a therapeutic tool) that can control and produce material in the form of microfibers in the intravascular environment was also introduced.
Global Interventional Intracranial Aneurysm Devices Market – Restraints
The increasing number of product recalls by regulatory authorities is expected to hamper the growth of the global interventional intracranial aneurysm market over the forecast period. For instance, in September, 2021, the U.S. Food and Drug Administration issued a class 1 recall for two Medtronic products: Pipeline Flex Embolization Device and Pipeline Flex Embolization Device with Shield Technology. Class 1 recalls are the most serious since using these products could result in fatal or serious injury. Moreover, on January 04, 2022, Balt USA, LLC, a manufacturer of neurovascular devices, initiated a class 2 device recall of Optima Coil System, a neurovascular embolization device, due to a label/pouch mix-up, erroneous shelf carton label or pouch label as a result of insufficient manufacturing line clearance. Following this labelling error, there is a chance that the wrong coil size may be accidentally chosen for surgery, which might lead to complications such as vessel damage, the inability to use the device to treat the aneurysm, or even its rupture.
Global Interventional Intracranial Aneurysm Devices Market – Regional Analysis
On the basis of region, the global interventional intracranial aneurysm devices market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa.
Among regions, Europe interventional intracranial aneurysm devices market is expected to hold a dominant position during the forecast period, owing to the introduction of technologically advanced products by key players in the interventional intracranial aneurysm market that has been driven by the rising incidence of cerebral aneurysms in the region. For instance, in October 2019, Per-flow Medical, a company that develops solutions for complex neurovascular treatments with presence in Europe, received the CE mark approval for its Cascade Agile. At the time of coil embolization, this cascade agile improves control for tortuous and distant artery morphology. The system's distinctive net construction ensures steady blood flow while coiling and treating brain aneurysms. For the treatment of cerebral aneurysms and acute ischemic stroke, this medication is heavily marketed in Europe. Therefore, it is projected that these improvements will fuel the market expansion during the forecast period.
Furthermore, North America is also estimated to witness significant growth in the global interventional intracranial aneurysm devices market owing to the presence of well-established healthcare facilities, increasing funding, etc. are expected to drive the market growth in North America over the forecast period. For instance, Fluid Biomed, a medical device start-up company in Calgary, Alberta, Canada, announced the completion of its US$ 4.7 million seed fundraising round and the appointment of two new directors to its board of directors. In the company's oversubscribed funding round, METIS Innovative of Miami, Florida, and ShangBay Capital of Palo Alto, California, collaborated as co-leads. This funding will accelerate the company’s growth and the development of a novel bio-absorbable stent to treat brain aneurysms.
Figure 2: Global Interventional Intracranial Aneurysm Devices Market (US$ Bn), by Region, 2022
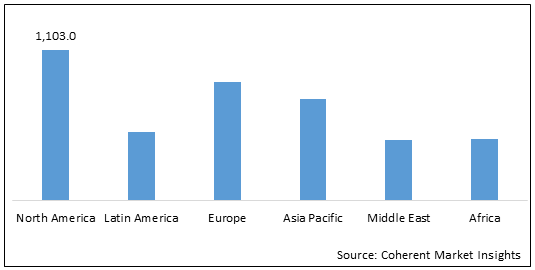
To learn more about this report, Download Free Sample
Global Interventional Intracranial Aneurysm Devices Market – Competitive Landscape
Major players operating in the global interventional intracranial aneurysm devices market include MicroPort Scientific Corporation, B. Braun SE, Stryker, MicroVention Inc., Johnson & Johnson Private Limited, Terumo Corporation, Penumbra, Inc., KANEKA CORPORATION, Perflow Medical Ltd, phenox GmbH, Evasc, ASAHI INTECC CO., LTD., Acandis GmbH, Imperative Care, Medtronic.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients