Interventional cardiology is the branch of cardiology that specifically deals with catheter-based treatment of heart diseases, such as congenital heart defect (CHD), coronary artery disease, and aneurysm. This involves insertion of the catheter into femoral artery or any large peripheral artery or vein and through catheter whole procedure is performed by using fluoroscopy.
Statistics:
The global interventional cardiology devices market is estimated to account for US$ 27,745.6 Mn in terms of value in 2020 and is expected to reach US$ 45,754.1 Mn by the end of 2027.
Global Interventional Cardiology Devices Market: Drivers
High prevalence of cardiovascular diseases is expected to propel growth of the global interventional cardiology devices market over the forecast period. For instance, according to the American Heart Association's Heart and Stroke Statistics 2019 Update, around 48% of all adults in the U.S. suffered from some type of cardiovascular disease in 2016.
Moreover, increasing demand for minimally invasive procedures is also expected to aid in growth of the global interventional cardiology devices market. Minimally invasive surgeries suit the patient’s preferences due to the following factors:
Statistics:
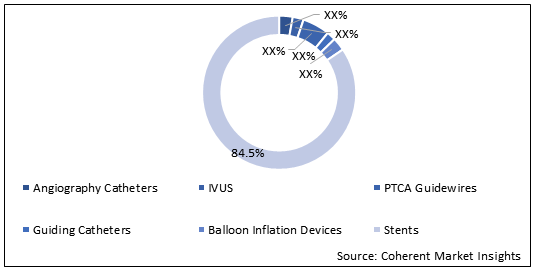
Stents held dominant position in the global interventional cardiology devices market in 2019, accounting for 84.5% share in terms of value, followed by PTCA Guidewires and Angiography Catheters, respectively.
Figure 1. Global Interventional Cardiology Devices Market Share (%), by Value, by Device, 2019
To learn more about this report, Download Free Sample
Global Interventional Cardiology Devices Market: Restraints
Complications associated with interventional cardiology procedures are expected to hinder growth of the global interventional cardiology devices market. Interventional cardiology surgeries are associated with complications as listed below:
Moreover, stringent approval norms and government regulations are also expected to limit the market growth. Interventional cardiology devices are categorized as high risk class III medical devices by the FDA and CE. This complicates the device approval process and makes the approval process long, stringent and expensive.
Interventional Cardiology Devices Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
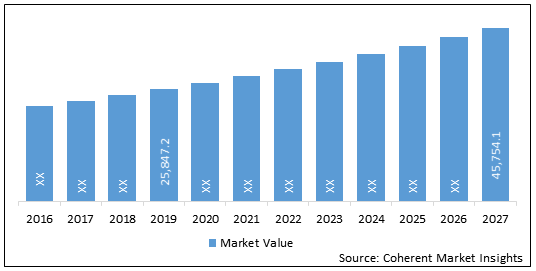
| Base Year: | 2019 | Market Size in 2019: | US$ 25,847.2 Mn |
| Historical Data for: | 2016 to 2019 | Forecast Period: | 2020 to 2027 |
| Forecast Period 2020 to 2027 CAGR: | 7.4% | 2027 Value Projection: | US$ 45,754.1 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Abbott Laboratories, Terumo Medical Corporation, Boston Scientific Corporation, Cardinal Health, Medtronic, Cook Medical, SINOMED, Biotronik SE & Co. KG, and B. Braun Melsungen AG. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Interventional Cardiology Devices Market: Opportunities
Increasing geriatric population is expected to offer lucrative growth opportunities for players in the global interventional cardiology devices market. For instance, according to the U.S. Census Bureau, the U.S. geriatric population is expected to reach 77 million by 2034.
Moreover, adoption of remote PCI procedure is also expected to aid in growth of the global interventional cardiology devices market. For instance, in December 2018, five patients located at the Apex Heart Institute in Ahmedabad, India, underwent an elective PCI procedure from a distance of roughly 20 miles (32km) away, using CorPath technology.
The global interventional cardiology devices market was valued at US$ 25,847.2 Mn in 2019 and is forecast to reach a value of US$ 45,754.1 Mn by 2027 at a CAGR of 7.4% between 2020 and 2027.
Figure 2. Global Interventional Cardiology Devices Market Value (US$ Mn), and Y-o-Y Growth (%), 2019-2027
To learn more about this report, Download Free Sample
Market Trends/Key Takeaways
Increasing number of PCI procedures is expected to aid in growth of the global interventional cardiology devices market. For instance, according to a report by National Intervention Council , India, and Apollo Health City, India, published in April 2019, a total of 3,87,416 PCI procedures were performed in 705 centers in India in 2017, which accounted for a 3.7% growth compared to that in 2016.
Major players in the global interventional cardiology devices market are focused on approval and launch of new products to expand their product portfolio. For instance, in April 2018, Terumo Corporation, a Tokyo-based medical device company, received CE mark for Ultimaster TANSEI drug-eluting stent.
Global Interventional Cardiology Devices Market: Competitive Landscape
Major players operating in the global interventional cardiology devices market include, Abbott Laboratories, Terumo Medical Corporation, Boston Scientific Corporation, Cardinal Health, Medtronic, Cook Medical, SINOMED, Biotronik SE & Co. KG, and B. Braun Melsungen AG.
Global Interventional Cardiology Devices Market: Key Developments
Major players in the global interventional cardiology devices market are focused on R&D to expand their product portfolio. For instance, in July 2019, SINOMED completed enrollment in the PIONEER III randomized, controlled clinical trial to assess the safety and effectiveness of the BuMA Supreme DES in order to obtain key regulatory approvals for the BuMA Supreme Drug-Eluting Stent (DES) System.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients