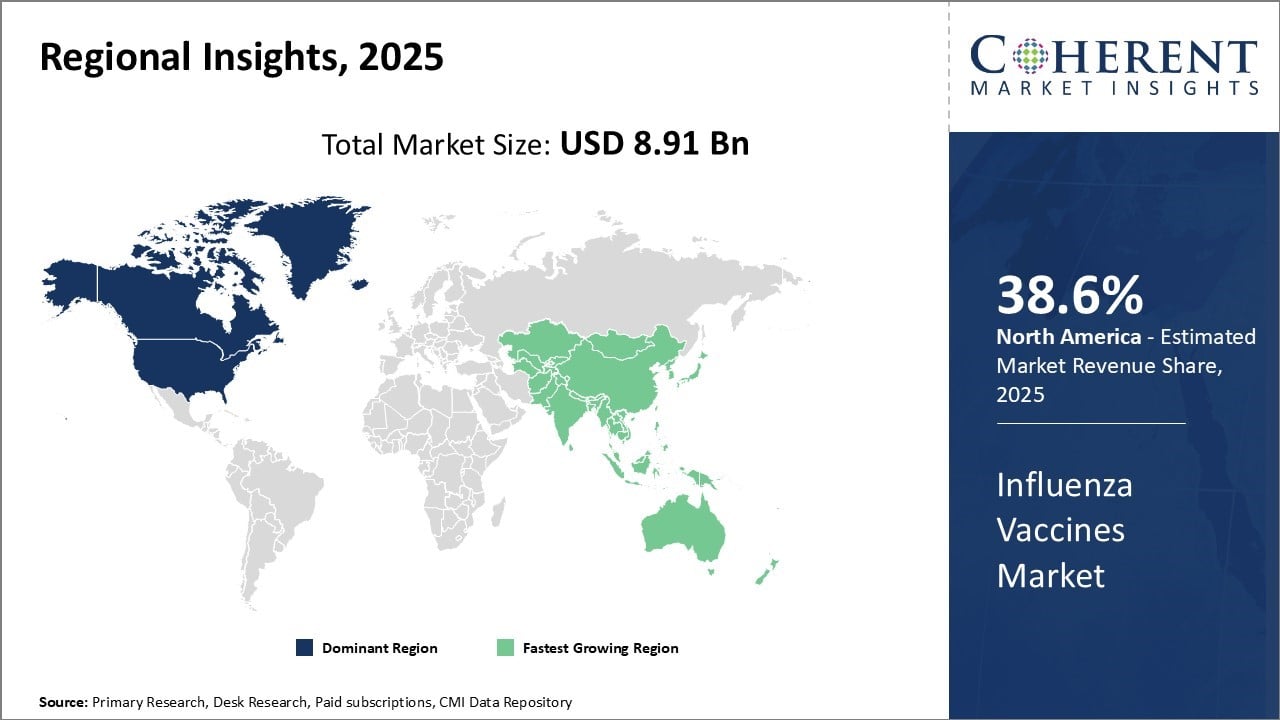
The influenza vaccines market is estimated to be valued at USD 8.91 Bn in 2025 and is expected to reach USD 14.59 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 7.3% from 2025 to 2032.
To learn more about this report, Download Free Sample
Influenza is a contagious respiratory infection caused due to influenza virus. Influenza is marked by fever, cough, muscles and joint pain, headache, and severe weakness. Illness due to influenza ranges from mild to severe, occasionally leading to death. Vaccines are recommended for the prevention of influenza infections. Influenza vaccine can be inactivated influenza vaccine, adjuvant inactivated influenza vaccine, live attenuated influenza vaccine or recombinant influenza vaccine. The rapid spread of influenza virus epidemics worldwide is propelling demands for the development of efficient influenza vaccine. Strong product pipeline is the key factor driving growth of the market during the forecast period.
In February 2025, Zydus Lifesciences introduced VaxiFlu‑4, India’s first quadrivalent inactivated influenza vaccine, covering two A strains and two B strains, in accordance with WHO recommendations. Developed at Zydus’s Vaccine Technology Centre in Ahmedabad and cleared by the Central Drug Laboratory, VaxiFlu‑4 offers broader annual protection against influenza.
|
Current Events |
Description and its impact |
|
Moderna Advances mRNA-Influenza Vaccine with Positive Phase 3 Results
|
|
|
UAE Deploys National Influenza Awareness Campaign for 2024–2025 Season
|
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Hospitals place a premium on influenza vaccines as essential for infection control and patient safety. They stress the need for early availability and high-efficacy products, particularly among vulnerable populations. Some of them mention difficulty in handling vaccine inventory in those uncertain flu seasons and indicate that better consistency in strain match is needed.
Clinics welcome the presence of quadrivalent vaccines and preservative-free versions, which improve patient acceptability. Several indicate that awareness campaigns boost in-store traffic for flu vaccination. Yet, smaller clinics occasionally face cold chain supply issues due to distance and low reimbursement rates in some areas.
Retail pharmacies show high demand for flu vaccines attributed to convenience and long hours. They emphasize advantages of single-dose products for speed and safety. Pharmacists prefer digital means for vaccine tracking and scheduling appointments, but mention high initial expenses in inventory and storage as constraints for smaller outlets.
Government institutions see influenza vaccination as a public health mainstay. They appreciate bulk purchasing discounts and WHO-matched strain coverage but frequently have problems with supply chain lags, particularly in rural settings. There is also a call for AI-enhanced outbreak surveillance to streamline distribution.
Scientists and institutions also find the existing vaccines useful for epidemiological studies and trials. They promote more funding for mRNA and universal flu vaccine research, and are interested in data-sharing collaborations with pharma companies for augmenting real-time monitoring of vaccine efficacy.
To learn more about this report, Download Free Sample
The vaccine type segment includes inactivated and live attenuated. Inactivated segment is estimated to hold 90.1% of the market share in 2025. Inactivated vaccines are created by growing influenza viruses in eggs or cells and then inactivating them with heat or chemicals. This process kills the viruses but keeps their antigens intact to produce an immune response in the body.
Compared to live attenuated vaccines, inactivated vaccines are much safer as they cannot replicate or cause infection. They are also considered more suitable for high-risk groups like pregnant women, the elderly and people with weakened immune systems.
In addition, inactivated vaccines have an established safety track record and longer shelf-life as they do not require viral growth and special storage conditions like refrigeration. Healthcare providers prefer recommending these vaccines to their patients knowing they would not face complications arising from using a live vaccine. Their advantages have firmly established inactivated vaccines as the preferred type for annual seasonal influenza vaccination programs worldwide.
The valency segment includes quadrivalent and trivalent. Quadrivalent segment contributes the highest share of the influenza vaccines market and is estimated to hold 91.9% of the market share in 2025. Quadrivalent vaccines target two strains of influenza A viruses and two influenza B virus lineages, whereas trivalent vaccines cover only one B virus lineage.
Experts have observed that influenza B viruses evolve more rapidly than influenza A viruses and can alternate between two distinct genetic lineages within a season. This can lower the effectiveness of trivalent vaccines which cover only one B lineage. Quadrivalent vaccines offer broader protection as they guard against viruses from both B lineages, thereby reducing the likelihood of influenza disease and spread during mismatched seasons when a different B lineage predominates.
Their enhanced coverage matches the current circulating strains in every season more closely and eliminates concerns over B lineage uncertainty. With increasing awareness about these factors, more healthcare providers and patients are opting for quadrivalent vaccines to gain complete defense against all major influenza strains.
The route of administration segment includes injection and nasal spray. Injection contributes the highest share of the influenza vaccines market and is estimated to hold 84.7% of the market share in 2024. Injected influenza vaccines are administered via intramuscular or subcutaneous routes into the arm or upper leg muscles.
This direct introduction of vaccine antigens into the body enables robust interaction with immune cells and strong activation of both humoral and cellular immunity. In contrast, nasal spray vaccines must first overcome biological barriers in the nasal mucosa before antigen exposure. As a result, injected formulations trigger higher antibody responses that last longer than nasal vaccines.
In addition, healthcare payers prefer injection over nasal sprays due to better data on safety, efficacy, and cost-effectiveness with decades of use. Needle-based delivery also better controls the exact dose received. While nasal sprays offer non-invasive delivery, their inconsistent immune responses have prevented widespread uptake, restricting this administration route to a smaller segment in the influenza vaccines market.
To learn more about this report, Download Free Sample
North America has dominated the global influenza vaccines market for many years due to strong healthcare infrastructure and awareness among people regarding the importance of vaccination and is estimated to hold 38.6% of the market share in 2025.
The U.S. accounts for the largest share within the region owing to the presence of major vaccine manufacturers and adoption of mandatory vaccination programs by healthcare facilities. Several manufacturers have their headquarters in the U.S. and undertake large scale production to meet domestic demand. In addition, vaccination rates have been steadily rising both among pediatric and adult populations.
Asia Pacific region is emerging as the fastest growing market for influenza vaccines worldwide. Expanding healthcare infrastructure, growing per capita healthcare expenditure and increasing focus of governments on immunization programs are some key factors driving market growth.
Countries like China, India and Japan are at the forefront due to their large population base and initiatives by public health organizations to spread awareness. Vaccine sales are rising sharply in Asia driven by changing lifestyles and growing cases of influenza-related illnesses in recent years.
The United States has the biggest market for influenza vaccines, driven by high awareness, annual public vaccination campaigns, and robust organizational backing from the CDC and FDA. Vaccines are widely given in workplaces, hospitals, and pharmacies, with private and public healthcare systems driving high demand. The country is also a hotbed for innovation, with the likes of Sanofi, Moderna, and GSK developing next-generation and combination flu-COVID vaccines.
The UK has a strong influenza immunization program within its National Health Service (NHS). It conducts annual flu vaccination campaigns for vulnerable groups such as the elderly, young children, and healthcare workers. The active engagement of the government through bulk purchasing as well as free vaccines for specified groups helps achieve mass coverage. The nation also prioritizes data-informed policymaking through institutions such as Public Health England.
Germany is an important player in the European flu vaccine market due to its strong healthcare system and emphasis on vaccinating patients with chronic illnesses and the elderly. The government works closely with pharma companies to drive availability and education. Germany also encourages vaccine development through its biotech cluster and university partnerships.
Japan has a significant number of elderly people and gives a great deal of importance to flu vaccination as part of its national health policy. Government-organized annual campaigns and school-based programs have led to long-term levels of coverage. Support by regulation for domestic and imported vaccine options in Japan has also helped to ensure stable market demand.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 8.91 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.3% | 2032 Value Projection: | USD 14.59 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
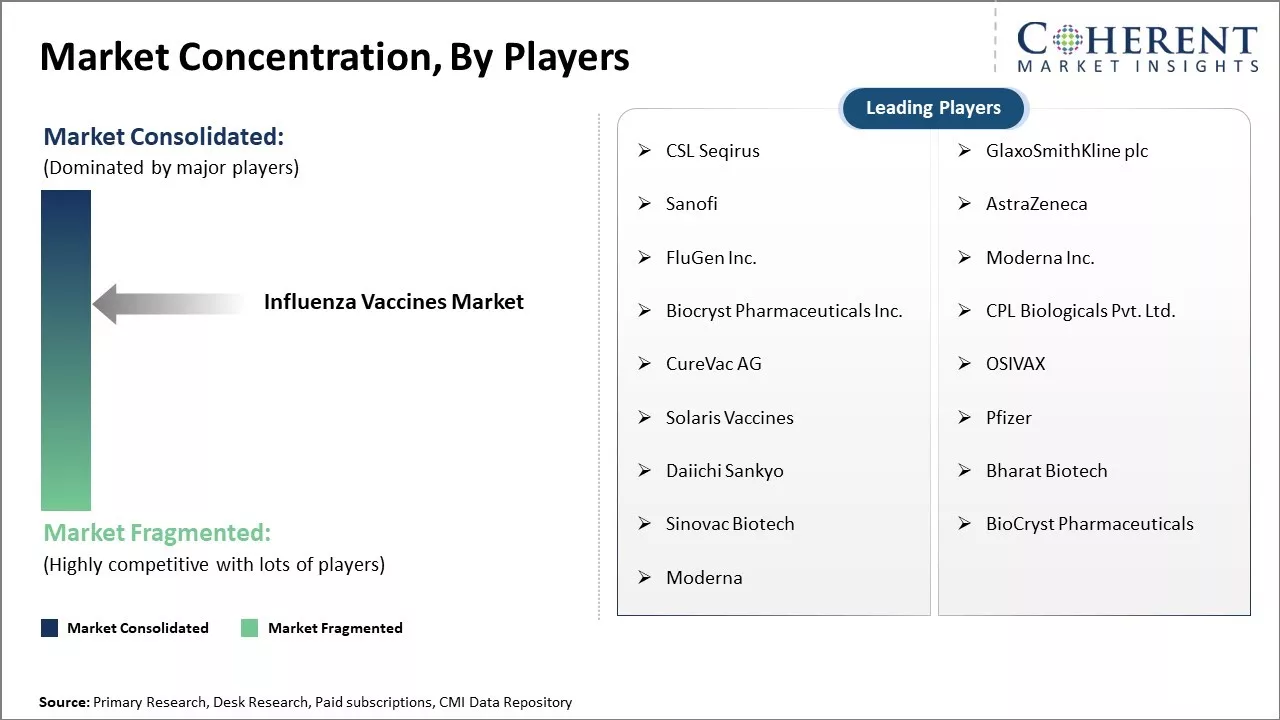
CSL Seqirus, GlaxoSmithKline plc, Sanofi, AstraZeneca, FluGen Inc., Moderna Inc., Biocryst Pharmaceuticals Inc., CPL Biologicals Pvt. Ltd., CureVac AG, OSIVAX, Solaris Vaccines, Pfizer, Daiichi Sankyo, Bharat Biotech, and Sinovac Biotech |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
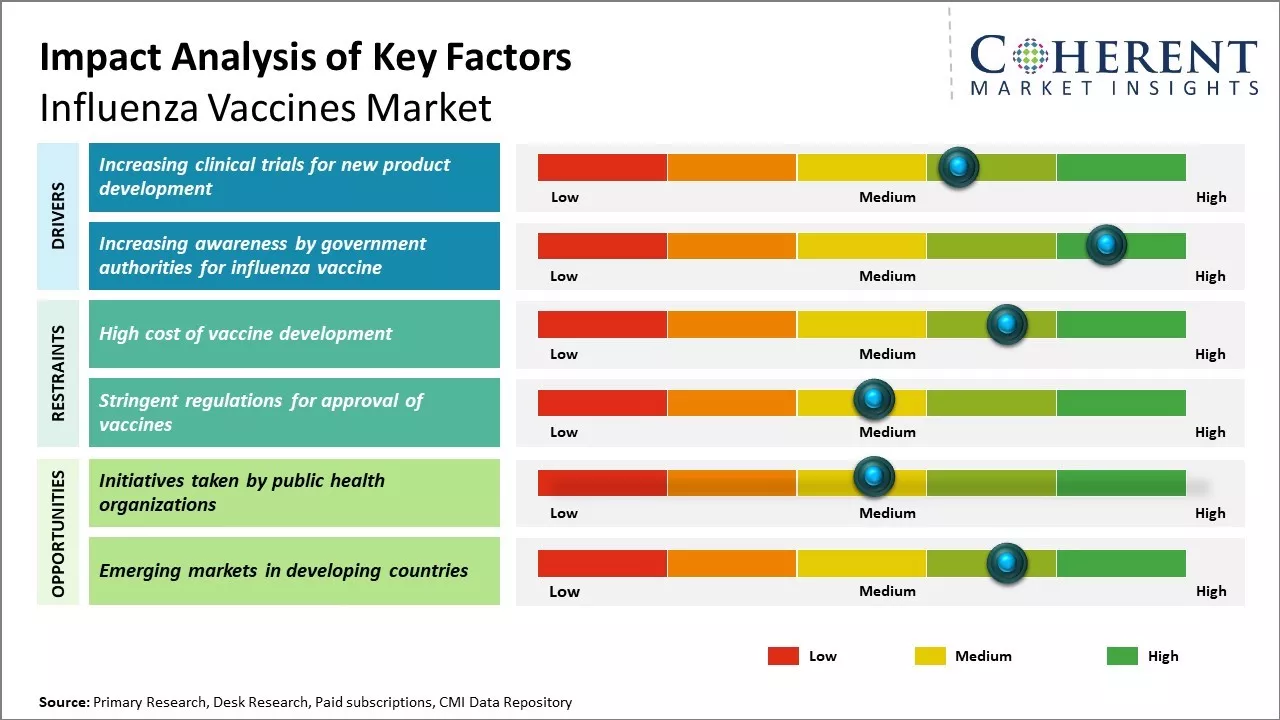
Increasing clinical trials is a key factor boosting the influenza vaccines market share. As flu viruses mutate rapidly, pharmaceutical companies are investing in advanced R&D to develop more effective and longer-lasting vaccines. These trials validate new formulations, delivery methods, and adjuvants, ensuring safety and efficacy across diverse populations. Regulatory approvals gained through clinical testing accelerate global distribution, especially in emerging markets. Trials also support innovation in universal flu vaccines and combination therapies. With rising public health awareness and demand for seasonal protection, clinical trials play a vital role in expanding the reach and reliability of influenza vaccines worldwide
Increasing awareness by government authorities is significantly boosting influenza vaccines market demand. Public health campaigns, such as the UK's “Get Winter Strong,” highlight the risks of flu-related complications and encourage vaccination among vulnerable groups. These initiatives improve public understanding of vaccine benefits, drive higher immunization rates, and reduce seasonal outbreaks. Governments also collaborate with healthcare providers to ensure widespread access and affordability. Educational outreach, media messaging, and school-based programs further reinforce the importance of annual flu shots. As awareness grows globally, especially in aging populations and high-risk groups, demand for influenza vaccines continues to rise, expanding market reach and impact. For instance, in September 2024, the NHS, alongside the UK Health Security Agency and the Department of Health and Social Care, launched its “Get Winter Strong” campaign. Public messaging emphasized that nearly 18,000 flu-related deaths occurred in England across the 2022–2024 seasons, urging eligible groups—especially the elderly, pregnant women, and the chronically ill—to get vaccinated.
Public health organizations globally are actively promoting influenza vaccination, significantly influencing the influenza vaccines market forecast. Through targeted awareness campaigns and educational programs, they emphasize the importance of annual flu shots, especially for vulnerable populations. These initiatives have led to increased vaccination rates, improved public understanding, and stronger demand. As governments continue to invest in outreach and preventive healthcare, the market is expected to grow steadily, with expanded access and innovation driving future developments in influenza vaccine distribution and uptake.
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients