Infectious Disease Therapeutics Market Size and Forecast – 2025 – 2032
The Global Infectious Disease Therapeutics Market size is estimated to be valued at USD 74.8 billion in 2025 and is expected to reach USD 116.5 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 6.8% from 2025 to 2032.
Global Infectious Disease Therapeutics Market Overview
Infectious disease therapeutics include antiviral, antibacterial, antifungal, and antiparasitic drugs designed to target and eliminate pathogenic organisms. These products act through various mechanisms such as inhibiting microbial replication, strengthening immune response, or preventing pathogen binding to host cells. Modern therapeutics include monoclonal antibodies, mRNA-based antivirals, and next-generation antibiotics resistant to bacterial evolution. Formulations are being optimized for improved efficacy, reduced side effects, and broader spectrum coverage against emerging infectious agents.
Key Takeaways
The antibiotic subsegment, owning 42% market share, remains the largest within the Infectious Disease Therapeutics market, propelled by persistent bacterial infection burdens.
Within disease indications, respiratory infections therapeutic demand surged significantly due to recurrent viral outbreaks, highlighting an enduring clinical focus.
North America dominates with a robust industry share, attributed to advanced R&D infrastructure and regulatory facilitation, hosting key market players responsible for substantial market revenue.
Asia Pacific exhibits the fastest market growth, backed by expanding healthcare infrastructure and strategic government initiatives aimed at infectious disease control and accessibility enhancement.
Infectious Disease Therapeutics Market Segmentation Analysis
To learn more about this report, Download Free Sample
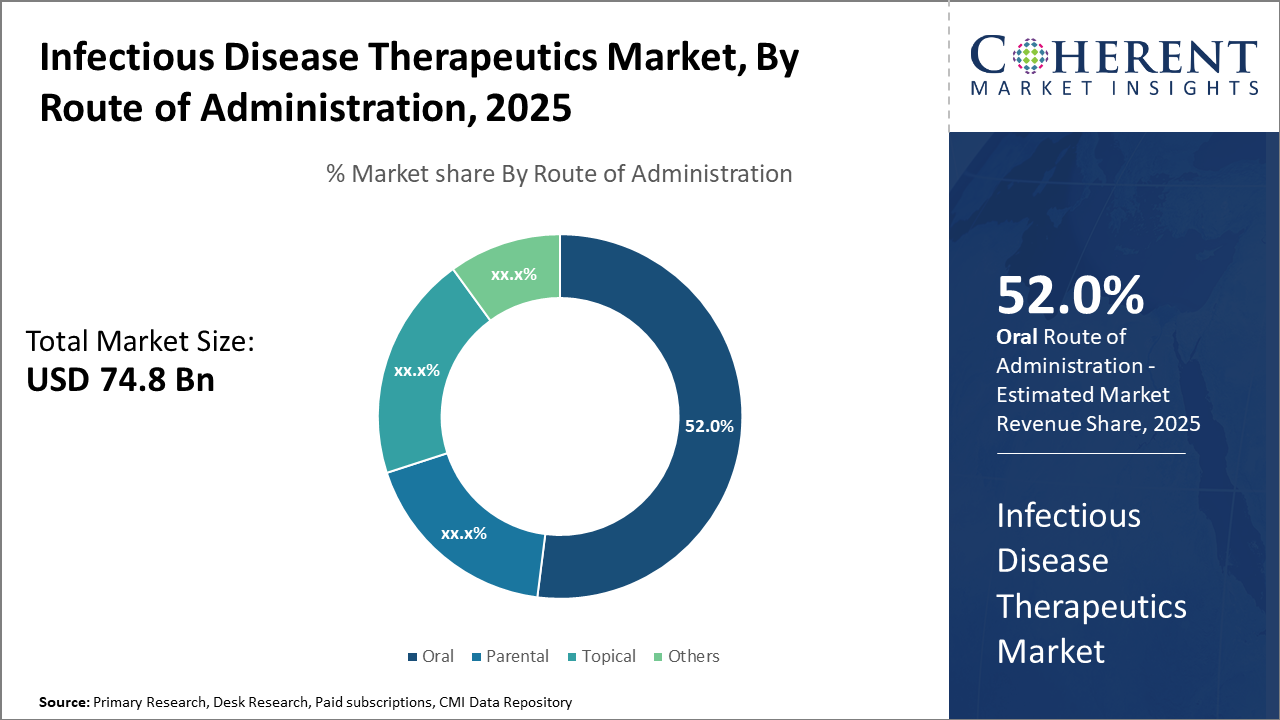
Infectious Disease Therapeutics Market Insights, By Route of Administration
Oral therapeutics dominate the market share at 52%, due to ease of administration and patient compliance, enabling broad outpatient care. The Parenteral segment is the fastest-growing, reflecting a shift toward injectable biologics and vaccines, enabling sustained drug delivery and enhanced efficacy. Topical formulations are used primarily for localized infections, maintaining a stable demand. The Others segment includes inhalation and novel delivery systems, emerging gradually as technological advances permit targeted site-specific treatments.
Infectious Disease Therapeutics Market Insights, By Therapeutic Type
Antibiotics dominate the market share with 42%, due to widespread bacterial infections and rising resistance, leading to sustained demand for next-generation antibiotics with novel action modes. Antivirals are the fastest-growing subsegment, fueled by heightened viral outbreaks and a surge in demand for hepatitis and HIV therapies. Antifungals represent a niche but essential segment addressing opportunistic infections, showing steady growth driven by immunocompromised patient populations. Vaccines contribute substantially to preventive strategies, with rapid advancements in vaccine technology such as mRNA-based platforms.
Infectious Disease Therapeutics Market Insights, By Disease Indication
Respiratory Infections dominate due to recurrent influenza and COVID-related complications, driving substantial product demand and research focus. Sexually Transmitted Infections are the fastest growing subsegment as increasing awareness and screening promote therapeutic adoption, especially for HIV and syphilis. Bloodborne Infections maintain a vestigial but consistent demand, particularly hepatitis B and C treatments. Gastrointestinal Infections, including Clostridium difficile infections, are gaining attention due to rising incidence in healthcare settings. Others encompass opportunistic infections like malaria, with region-specific impact.
Infectious Disease Therapeutics Market Trends
Recent market trends reflect a significant push toward innovative therapeutic modalities, such as mRNA and monoclonal antibodies, with a 25% increase in clinical adoption noted in 2024.
Additionally, AI integration has begun to dominate drug discovery phases, reducing both time and cost investments.
These technological advances are supported by expanding global clinical trial activities, particularly in infectious diseases with unmet medical needs.
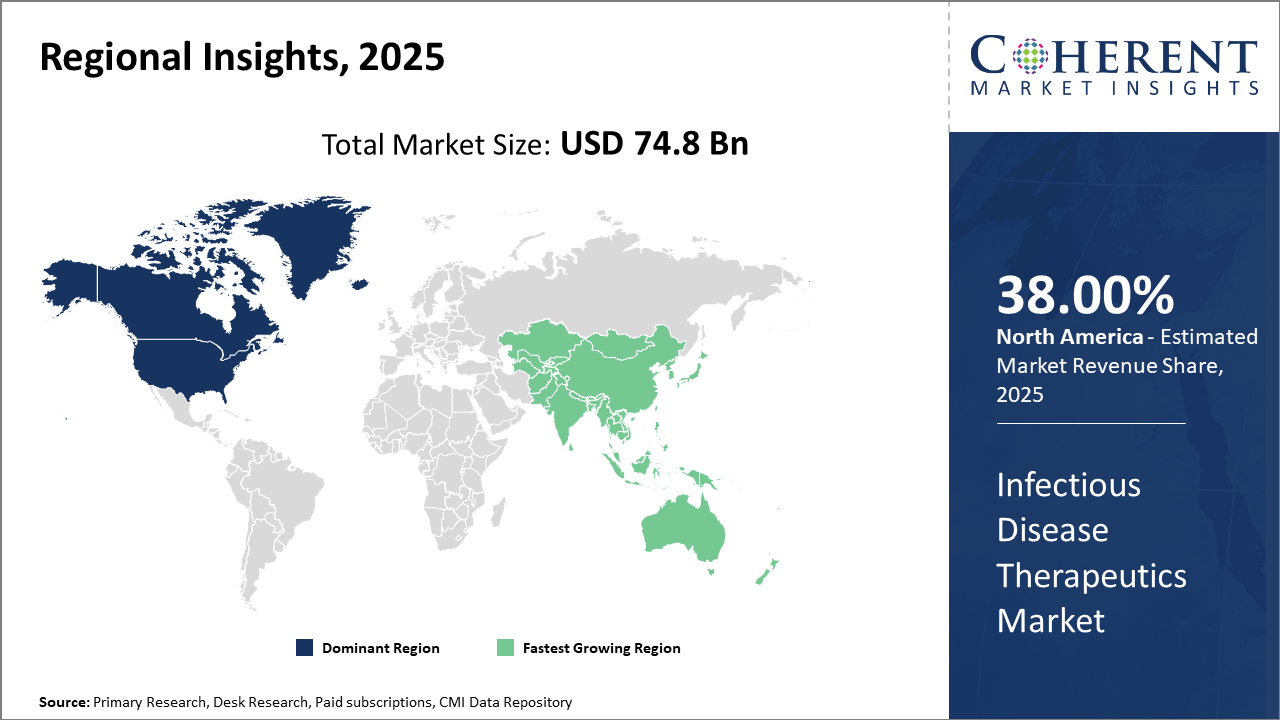
Infectious Disease Therapeutics Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Infectious Disease Therapeutics Market Analysis and Trends
North America dominates the market share at 38%, due to strong R&D infrastructure, comprehensive healthcare expenditure, and regulatory support. The U.S. accounted for approximately 40% of the total industry share in 2024, driven by significant investments from leading market companies and robust clinical development pipelines facilitating business growth.
Asia Pacific Infectious Disease Therapeutics Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth, with a CAGR surpassing 8% during 2025–2032. Factors accelerating this expansion include government healthcare initiatives, increasing prevalence of infectious diseases, and growing pharmaceutical manufacturing capabilities in China and India. Market companies such as Sanofi and Moderna have strengthened their presence in this region, contributing to increased market revenue.
Infectious Disease Therapeutics Market Outlook for Key Countries
USA Infectious Disease Therapeutics Market Analysis and Trends
The USA’s Infectious Disease Therapeutics market is characterized by groundbreaking innovation and substantial capital deployment in R&D, with over 30% of the global clinical trials conducted domestically. Major players like Pfizer and Merck contribute heavily to market revenue through the timely launch of antiviral and antibiotic products. Government initiatives accelerating pandemic preparedness and antimicrobial stewardship further propel industry size and market share.
India Infectious Disease Therapeutics Market Analysis and Trends
India's market has witnessed rapid expansion due to rising infectious disease burdens and enhanced healthcare accessibility. Domestic pharmaceutical firms combined with multinational corporations are advancing generic and novel therapeutics, fueling competitive market growth. The country’s policy focus on manufacturing self-reliance and improved drug regulations has resulted in a 15% increase in market revenue in 2024, positioning it as a critical hub for Infectious Disease Therapeutics.
Analyst Opinion
Supply and Production Scale: The rise in infectious disease incidences worldwide has precipitated a marked increase in therapeutic production capacities. For instance, global vaccine production volume expanded by nearly 15% in 2024 alone due to enhanced manufacturing facilities in emerging economies. This supply-side amplification supports the growing market share, particularly in regions grappling with disease outbreaks.
Demand Heterogeneity Across Regions: Demand-side indicators reflect a surge in therapeutic imports in regions like Latin America and the Asia Pacific, where treatment accessibility remains a pressing issue. Import volumes of antiviral agents in Brazil increased by 18% year-over-year in 2024, exemplifying a diverse use case spectrum that fuels market revenue and business growth.
Pricing Dynamics and Reimbursement Patterns: Pricing strategies have exhibited significant variation, with specialty infectious disease therapeutics commanding premium rates, influenced by the novel mechanisms of action. In 2025, the average price per treatment course for novel antifungal agents rose by 8%, underpinned by increased R&D investment and regulatory approvals.
Micro-market Segmentation Impact: Within niche therapeutic categories, such as monoclonal antibodies for infectious diseases, the market witnessed a 25% uptick in clinical adoption in 2024, catalyzing overall market growth. Rapid innovation cycles and targeted treatment approaches are pivotal drivers enhancing market insights and analytical depth.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 74.8 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.8% | 2032 Value Projection: | USD 116.5 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Pfizer Inc., GSK plc, Merck & Co., Inc., Johnson & Johnson, Roche Holding AG, Novartis AG, Sanofi S.A., Bristol-Myers Squibb Company, AbbVie Inc., Bayer AG, Takeda Pharmaceutical Company Limited, AstraZeneca plc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Infectious Disease Therapeutics Market Growth Factors
The increasing prevalence of multidrug-resistant pathogens remains a primary growth driver, compelling investments in novel antibiotics and antivirals. Around 700,000 deaths were attributed to antimicrobial resistance in 2024, underscoring urgent therapeutic demand. Regulatory supports and fast-tracking of infectious disease drugs by agencies such as the FDA have shortened development timelines, encouraging innovation pipelines. Additionally, rising healthcare expenditure in emerging regions has broadened patient access to advanced therapeutics, reflected by a 10% increase in infectious disease treatment centers in Asia Pacific in 2025. Furthermore, digital health integration, such as telemedicine for infectious disease monitoring, improves early diagnosis and treatment adherence, enhancing market revenue and industry share.
Infectious Disease Therapeutics Market Development
In June 2025, Merck received U.S. FDA approval for ENFLONSIA™ (clesrovimab-cfor), a monoclonal antibody developed to prevent lower respiratory tract disease caused by respiratory syncytial virus (RSV) in infants. ENFLONSIA is indicated for newborns and infants entering or during their first RSV season, offering long-lasting protection through a single intramuscular dose. The approval marks a major milestone for Merck in pediatric infectious disease prevention, positioning ENFLONSIA as a key option alongside existing prophylactic antibodies and maternal immunization approaches.
In February 2025, AbbVie and Pfizer jointly announced the U.S. FDA approval of Emblaveo, a novel antibiotic combination therapy for the treatment of complicated intra-abdominal infections in adults. Emblaveo combines aztreonam-avibactam, designed to overcome resistance from metallo-β-lactamase-producing pathogens, offering a critical new treatment option against multidrug-resistant Gram-negative bacteria. The approval follows positive results from global Phase III clinical trials demonstrating non-inferiority compared to standard-of-care antibiotics in severe infections.
Key Players
Leading Companies of the Market
Pfizer Inc.
GSK plc
Merck & Co., Inc.
Johnson & Johnson
Roche Holding AG
Novartis AG
Sanofi S.A.
Bristol-Myers Squibb Company
AbbVie Inc.
Bayer AG
Takeda Pharmaceutical Company Limited
AstraZeneca plc
Several market companies have leveraged strategic collaborations to enhance their product pipelines. For example, in early 2025, Pfizer and BioNTech expanded their joint development agreement for next-generation antiviral vaccines, successfully accelerating candidate immunotherapies into Phase III trials. Similarly, Gilead Sciences’ acquisition of a smaller biotech firm specializing in RNA-based infectious disease therapeutics resulted in a 12% revenue surge within its infectious disease division in 2024.
Infectious Disease Therapeutics Market Future Outlook
The market is poised to transition toward next-generation therapies leveraging biotechnology, immunomodulation, and digital surveillance. The focus will shift to broad-spectrum antivirals, pathogen-specific biologics, and adaptive treatment models supported by AI-driven epidemiology. Enhanced collaboration between pharmaceutical firms and global health agencies will ensure rapid response to emerging infections. The growing emphasis on antimicrobial stewardship, rapid diagnostics, and pandemic preparedness will guide strategic investments. Markets in developing regions will see significant expansion due to vaccination programs, healthcare reforms, and global funding mechanisms.
Infectious Disease Therapeutics Market Historical Analysis
The infectious disease therapeutics market has a long and complex history shaped by global public health needs, scientific discovery, and epidemiological crises. From the antibiotic revolution of the 20th century to the rise of antiviral and immunotherapy solutions, the market evolved through cycles of innovation and resistance. Globalization, urbanization, and increased travel intensified disease transmission, prompting stronger investment in vaccine development and antimicrobial research. Public-private partnerships and international funding initiatives played pivotal roles in accelerating treatment availability for diseases such as HIV, tuberculosis, and malaria.
Sources
Primary Research interviews:
Epidemiologists
Infectious Disease Specialists
Pharmaceutical Scientists
Public Health Experts
Databases:
WHO Global Health Observatory
CDC Infectious Diseases Database
ClinicalTrials.gov
Magazines:
The Lancet Infectious Diseases
Gnn Medical News Today
PharmaVoice
BioWorld
Journals:
Journal of Infectious Diseases
Clinical Infectious Diseases
Emerging Infectious Diseases
Nature Medicine
Newspapers:
Hindustan Times (Health)
The Economic Times (Pharma)
The Guardian (Global Health)
The New York Times (Science)
Associations:
World Health Organization
Infectious Diseases Society of America
Global Virus Network
Centers for Disease Control and Prevention
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients