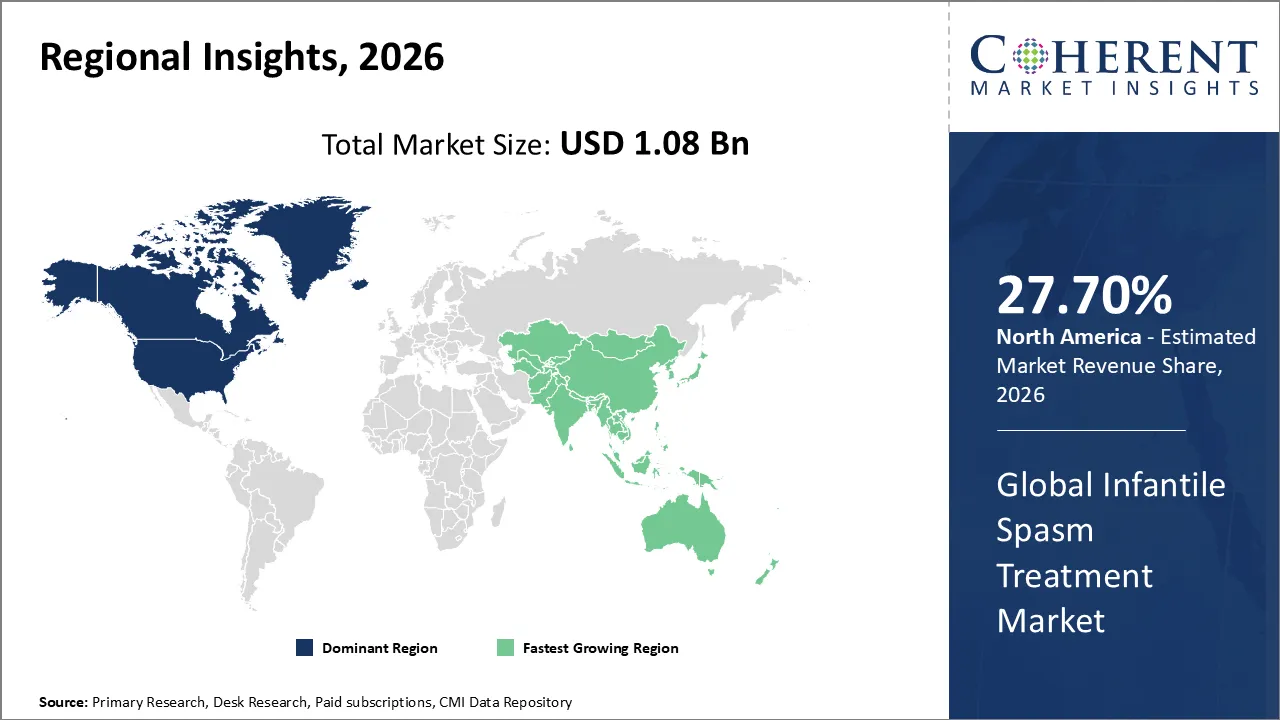
The Infantile Spasm Treatment Market is estimated to be valued at USD 1.08 Bn in 2026 and is expected to reach USD 1.67 Bn by 2033, exhibiting a compound annual growth rate (CAGR) of 4.1% from 2026 to 2033.
The Infantile Spasm Treatment Market is growing because more people are recognizing and diagnosing infantile spasms, especially in babies with neurological problems. Caregivers and doctors are using therapies more and more because people are becoming more aware of them, pediatric neurology is getting better, and early intervention programs are being used. Healthcare providers use both oral and injectable treatments, such as antiepileptic drugs and hormonal treatments. Better healthcare infrastructure, supportive regulations, and new drug formulations make these treatments easier to get, make sure that patients follow the rules, and improve clinical outcomes for babies with these conditions.
|
Current Events |
Description and its impact |
|
Regulatory and Policy Developments |
|
|
Technological Innovations and R&D Advances |
|
|
Regional Regulatory and Safety Standards Evolution |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Vigabatrin hold the largest market share of 34.4% in 2026. Vigabatrin is the most popular drug in the Infantile Spasm Treatment Market because it works so effectively, especially in babies with tuberous sclerosis complex, where it quickly lowers the number of seizures. Caregivers can deliver the medicine in pill form at home, which makes it easier to follow the instructions and more convenient. In some cases, professionals recommend vigabatrin as a first-line treatment because there aren't many alternative options and there is solid clinical evidence for it. Regulatory support, the availability of generics, and continual improvements to the formulation all make it simpler to access vigabatrin. This is a key component in improving long-term neurological outcomes for affected neonates.
For instance, Upsher-Smith Laboratories launched VIGADRONE® 500 mg tablets, a fully substitutable generic of Sabril®, and also offers VIGADRONE® 500 mg oral solution.
Oral acquired the prominent market share of 51.2% in 2026. The oral sector is the main driver of the Infantile Spasm Treatment Market because it offers a simple, non-invasive technique to control seizures in babies. Caregivers offer oral medications at home, which makes it easier for patients to stick to their treatment plans and cuts down on hospital visits. Age-appropriate formulations, such as liquid solutions and tablets that may be broken up, make it easier to get the right dose and follow the rules. As more people learn about infantile spasms, doctors are more likely to offer oral drugs because there aren't many other options. This speeds up the process of getting babies on these meds and helps them get better faster.
For instance, MSN Labs launched Viganext, a generic Vigabatrin powder for oral solution, to treat infantile spasm, a form of epilepsy in children.
To learn more about this report, Download Free Sample
North America dominates the overall market with an estimated share of 27.7% in 2026. The North America Infantile Spasm Treatment Market is growing because of better pediatric neurology facilities, early diagnostic programs, and more doctors and caregivers who know about the disorder. More and more healthcare practitioners are employing new oral and injectable medicines that are safe for kids. This makes treatments more effective and easier to stick with. Regulatory assistance, incentives for orphan pharmaceuticals, and comprehensive reimbursement frameworks make it easier for patients to receive drugs and encourage pharmaceutical development. Also, home-based and outpatient care models make it easier and safer for caregivers to deliver therapies. This means that more individuals use them, which leads to better long-term neurological outcomes for babies with infantile spasms.
For instance, in August 2024, Mallinckrodt plc launched the Acthar Gel Single-Dose Pre-filled SelfJect™ Injector, providing a new administration option for patients with chronic and acute inflammatory or autoimmune conditions, except for infantile spasms.
The Asia Pacific Infantile Spasm Treatment Market is expanding because more people are learning about neurological diseases and doctors are getting better at finding them. Healthcare providers are establishing more specialized pediatric clinics and infrastructure so that problems can be detected and addressed swiftly. Caregivers are giving kids more and more oral and injectable drugs that are made for youngsters. This makes it easier for them to remain with the therapy. Governments launch programs, give healthcare more money, and increase insurance coverage to make therapy easier to get. At the same time, drug companies come up with novel treatments that make people want to utilize them. This makes newborns with infantile spasms in the area do better in the clinic.
The US market for treating infantile spasms is growing because people are becoming more aware of seizure disorders in children and programs that encourage fast identification. Clinicians employ advanced healthcare infrastructure and specialized pediatric neurology institutes to deliver oral and injectable therapies that function well. Patients can acquire innovative treatments more easily because of regulatory incentives and full insurance coverage. At the same time, drug companies are producing medicines that are easier for youngsters to take so that they will stick to their drugs. Families are also giving therapies more often at home and in outpatient settings, which makes treatments more common and helps babies with infantile spasms have better long-term neurological results.
For instance, U.S. Based Pyros Pharmaceuticals announced a positive outcome for VIGZIP™ (vigabatrin oral solution) under the Decentralised Procedure (DCP) in Europe.
The China Infantile Spasm Treatment Market is growing because more people know about neurological diseases in children and doctors can now discover them more quickly. To make sure that problems are found and addressed right away, healthcare professionals set up specialist pediatric neurology units. More and more, caregivers are giving kids oral and injectable medicines that are made particularly for them. This helps kids remember to take their prescription and stick to their treatment plan. People can get treatments more easily now since the government is doing things to help, there is more money for healthcare, and there is more insurance coverage. At the same time, pharmaceutical companies are making novel medications in the US that are more likely to be used. These medications treat babies with infantile spasms all around the country.
Oral therapies are becoming increasingly popular in the market for infantile spasms because they are easy to take, don't need surgery, and can be done at home. Pediatric-friendly forms, like liquids and tablets that may be dissolved in water, make it easier to take the proper dose and stick to the treatment plan. Caregivers and doctors prefer oral options to needles since they are safer, easier to use, and don't require as many trips to the hospital. This makes them a key part of getting more newborns to start therapy and making them healthier.
Healthcare professionals stress the need of early detection of infantile spasms using EEG tests, neuroimaging, and genetic testing. Early diagnosis lets doctors start treatment right away, which lowers the risk of long-term neurological problems. Awareness initiatives aimed at parents and caregivers, together with regular monitoring in pediatric neurology centers, help find more instances and get people started on treatment right away. This shapes the market need for successful medicines.
Manufacturers may make safe, easy-to-give medicines that are right for babies and young children. Formulations that make it easier to provide the medicine, taste better, and lower the risk of dosing mistakes can make people more likely to take it and make caregivers happier. Companies may satisfy important unmet requirements, build brand loyalty, and increase adoption in hospitals, clinics, and home-care settings by focusing on drug delivery that is oriented on children.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 1.08 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 4.1% | 2033 Value Projection: | USD 1.67 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
H. Lundbeck A/S, Mallinckrodt Pharmaceuticals, Catalyst Pharmaceuticals, GW Pharmaceuticals plc, Retrophin, Inc., Valerion Therapeutics, Orphelia Pharma SA, Insys Therapeutics, Inc., and Anavex Life Sciences Corp. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients