India Intravenous Anesthetic Market is estimated to be valued at USD 77.7 Mn in 2025 and is expected to reach USD 96.9 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 3.2% from 2025 to 2032.
Analysts’ Views on India Intravenous Anesthetic Market:
Due to increasing prevalence of cardiovascular diseases, chronic diseases and spine disorders, which leads to the various surgeries. With the increase in number of surgeries, the growth of intravenous anesthetics gradually increases. For instance, according to an article published in Journal of Anaesthesiology Clinical Pharmacology in November 2022, a new type of anesthesia called "Ketofol" that can be used for patients with multiple sclerosis. The article explains how the anesthesia works and how it was used successfully for a patient undergoing a Magnetic Resonance Imaging (MRI)
India Intravenous Anesthetic Market – Driver
Change in lifestyle, rising number of chronic diseases and increase in geriatric population
Changing in the lifestyle which increases the obesity among the healthy volunteers along with the increase number of chronic diseases resulted into increase number of surgeries, which is expected to drive the India intravenous anesthetic market. For instance, in September 2022, AIIMS Delhi discusses the increase in knee replacement surgeries in India over the past five years, which is due to factors such as increased longevity and availability of expertise and infrastructure. More than 2. 5 lakh people undergo total knee replacement surgery in India every year which is almost 2.5 times the number of such procedures conducted annually.
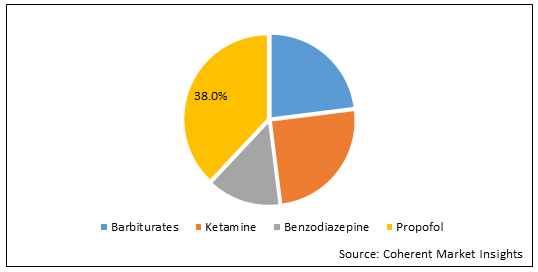
Figure. India Intravenous Anesthetic Market Share (%), by Product Type, 2025
To learn more about this report, Download Free Sample
India Intravenous Anesthetic Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others are facing problems with the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a negative impact on the intravenous anesthetics market. The pandemic had stressed up the healthcare systems in the world and cause decline in the general anesthesia due to cancelled or postponed elected surgeries. For instance, on February 12 2023, according to an article published, patients infected with COVID-19 may be at increased risk of perioperative complications, and anesthesiologists must carefully evaluate patients prior to surgery to minimize these risks.
India Intravenous Anesthetic Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 77.7 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 3.2% | 2032 Value Projection: | USD 96.9 Mn |
| Segments covered: |
|
||
| Companies covered: |
AstraZeneca, Plc., Fresenius Kabi, AbbVie Inc., Baxter International Inc., Braun Melsungen AG, Maruishi Pharmaceutical. Co., Ltd., Piramal Pharma Ltd., Hikma Pharmaceuticals, Inc., and Viatris Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
India Intravenous Anesthetic Market Segmentation:
The India intravenous anesthetic market report is segmented into by Type, and by End User
By Type, the market is segmented into Barbiturates, Ketamine, Benzodiazepines, Propofol and Others. Out of which, propofol segment is expected to hold a dominant position in the intravenous anesthetics market during the forecast period. For instance, in August 2025, according to the article published in Computational and Mathematical Methods in Medicine, reveals the effect of propofol intravenous anesthesia combined with press-needle therapy on analgesic effect during painless abortion. Propofol, an intravenous anesthetic is used for general anesthesia induction, monitored anesthesia management, and procedural sedation. It may be given as an infusion, a bolus, or a mix of the two. It can also be administered as part of an anesthesia maintenance technique called total intravenous anesthesia.
By End User, the market is segmented into Hospitals, Clinics and Others. Out of which, the hospital segment is expected to hold a dominant position in the intravenous anesthetics market during the forecast period and this is attributed to the increasing hospitals along with the increase in number of surgeries. For instance, according to article published in September 2020, the article estimated a total of 4642 surgeries were performed per year for a population of 88,273. Cataract (22.8%), Caesareans (3.8%), surgeries for fractures (3.27%) and hernia (2.86%) were the commonest surgeries. 44.2% of surgeries belonged to the essential surgeries.
Among all the segmentations, the type segment has the highest potential due to the increasing prevalence of surgeries with the chronic diseases over the forecast period. For instance, in October 2022, a phase IV clinical trial is carried out at Sir Ganga Ram Hospital in India to assess if total body weight should be used to determine the propofol dosage for obese patients getting total intravenous anesthesia. Propofol is an intravenous anesthetics used for general anesthesia induction, monitored anesthesia management, and procedural sedation.
India Intravenous Anesthetic Market: Key Developments
In January 2022, All India Institutes of Medical Sciences (AIIMS), New Delhi had implemented new digital surgery technology from ImmersiveTouch, a medical technology company building the digital surgery metaverse. Its Mission Rehearsal VR platform gives surgeons the ability to virtually plan and simulate each patient's unique anatomy in 3D, prior to entering the operating room.
On February 8 2023, ICU Medical Inc., a manufacturer and seller of innovative medical devices, announced its Plum 360 smart infusion system is the first to be recognized as the top-performing smart pump in both the EMR-Integrated and Traditional categories by KLAS Research, a global health care research firm. Plum 360 smart infusion system is the only smart infusion system to win the Smart Pump EMR-Integrated category.
In April 2022, Avet Pharmaceuticals Inc., a generic pharmaceutical company, announced the launch of its propofol injectable emulsion, USP 10 mg/mL, in 20, 50 and 100 ml Single Patient-Use Vials, an AB rated generic equivalent of DIPRIVAN (propofol) Injectable Emulsion USP, following its abbreviated new drug application approval from the U.S. Food and Drug Administration (FDA).
In July 2022, Datwyler, a manufacturer of elastomer components for pharmaceutical packaging, is expanding its portfolio with two new products to meet evolving patient demands. The new offerings include a 5/8 inch rigid needle shield (RNS) to address the rising need for prefilled syringes to contain vaccines for intramuscular injection, and isoprene rubber discs for infusion therapies.
India Intravenous Anesthetic Market: Key Trends
Better healthcare infrastructure, effective government policies can drive the growth of market
Due to improved healthcare infrastructure, effective government policies, a sizable base of multinational corporations, and an increase in genetic illnesses, the intravenous anesthetics market is expected to grow over the forecast period.
In September 2021, AIIMS New Delhi and India Medtronic Private Limited, a wholly owned subsidiary of Medtronic plc, announced the opening of a state-of-the-art surgical robotics training center at AIIMS, New Delhi. The center will provide surgeons with best-in-class training in robotic-assisted surgery. It is the first such center at AIIMS utilizing Medtronic’s HugoTM robotic-assisted surgery (RAS) system, which was first introduced in India.
India Intravenous Anesthetic Market: Restraint
Overdose of Anesthesia causing sudden death
Product recall due to visibility of entities in the sterile preparations a major restraint on the growth of the India intravenous anesthetic market. Moreover, the increase prevalence of overdose of anesthesia leads to the death of the individual cane lead to restrict the growth of intravenous anesthesia. For instance, in September 2022, Central Drugs Standard Control Organization (CDSCO), an India's national regulatory body for cosmetics, pharmaceuticals and medical devices gave a red alert on use of propofol injection, due to sudden death of 5 patients in Chandigarh as the propofol is administered to them prior to any big surgery.
Product recalls from the regulatory body
Certain factors such as potential breakdown in the inhaled preparation of surgical anesthetic is expected to hinder the growth of the India intravenous anesthetic market during the forecast period. To support this, company is planning to support this voluntarily recall by providing another batch manufacturing with no visible entities. A Letter to affected customers informing them of the product issue and potential risks to health is to be provided. For instance, in December, 2021, Getinge sent an Urgent Medical Recall Letter to affected customers informing them of the product issue and potential risks to health. Getinge is recalling the Vaporizer Sevoflurane Maquet filling due to the potential chemical breakdown of Sevoflurane, a general surgical anesthetic, which may result in inhalation and/or skin exposure to harmful chemicals. If this occurs, this may cause serious patient harms including irritation of the respiratory tract, lung edema (swelling caused by excess fluid), and severe hypocalcemia (condition in which the blood has too little calcium).
India Intravenous Anesthetic Market - Key Players
Major players operating in the India intravenous anesthetic market include AstraZeneca, Plc., Fresenius Kabi, AbbVie Inc., Baxter International Inc., Braun Melsungen AG, Maruishi Pharmaceutical. Co., Ltd., Piramal Pharma Ltd., Hikma Pharmaceuticals, Inc., and Viatris Inc.
*Definition: Intravenous anesthetics are a group of substances known as diverse and includes substances including barbiturates, benzodiazepines, opioids, ketamine, and propofol. Since they take effect within 20 seconds, intravenous anesthetics are frequently used to induce anesthesia. This method is frequently preferred to induction using an inhaling agent through a face mask. Because the duration of action would require continuous infusion to maintain anesthesia, intravenous drugs are not frequently utilized for maintaining anesthesia during surgical procedures because they are relatively slowly removed from the body. Intravenous anesthetics are used in the operating room to both induce and maintain general anesthesia, for preoperative sedation and anxiolysis, for procedures outside the operating room, and in the intensive care unit (ICU). In some patient populations, propofol-based TIVA is preferable to anesthesia maintenance with volatile anesthetics. To maximize the essential properties of the anesthetic and reduce toxicity, the combination of intravenous anesthetics can be customized for each patient and treatment.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients