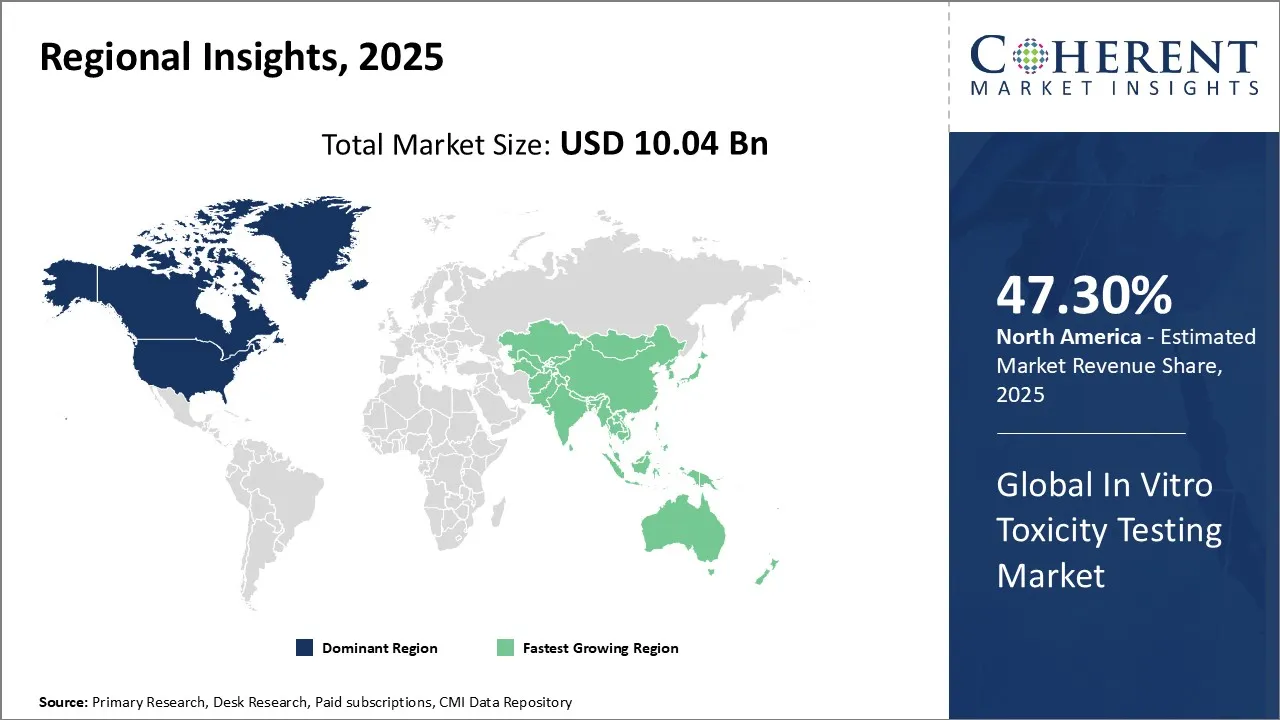
In Vitro Toxicity Testing Market is estimated to be valued at USD 10.04 Bn in 2025 and is expected to reach USD 24.36 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 13.5% from 2025 to 2032.
The in vitro toxicity testing market is witnessing robust demand due to advancements in cell-based assays and growing regulatory pressure to reduce animal testing. Increasing applications in pharmaceuticals, cosmetics, and chemical safety assessments are driving innovation in high-throughput and toxicogenomic technologies. The rise in chronic diseases and personalized medicine further fuels the in vitro toxicity testing market demand, as companies seek efficient, ethical, and predictive testing methods to accelerate product development and ensure consumer safety.
|
Current Event |
Description and its Impact |
|
Regulatory Shifts Toward Animal Testing Alternatives |
|
|
Technological Breakthroughs in Organ-on-Chip and AI Integration |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
In terms of type, the absorption segment is expected to lead the market with 48.7% share in 2025, due to its vital role in assessing drug permeability and bioavailability. It supports early-stage screening, reduces reliance on animal testing, and aligns with regulatory trends. Technological advances in cell models further enhance its accuracy and appeal across pharmaceutical and cosmetic industries.
For instance, in September 2025, CN Bio unveiled the PhysioMimix® Bioavailability Assay Kit: Human 18, a Gut/Liver-on-a-Chip solution designed to simulate human oral drug absorption and metabolism. This innovation enhances in vitro toxicity testing by offering a predictive, animal-free method for assessing drug bioavailability, supporting ethical and efficient pharmaceutical development.
In terms of end user, the pharmaceutical industry segment is expected to hold the largest share of the market in 2025, fueled by its demand for early-stage toxicity screening in drug development. Regulatory pressures and the shift toward cost-effective, non-animal testing methods enhance adoption. High-throughput screening and advanced cell-based assays are especially preferred, enabling faster, more predictive safety evaluations across the drug discovery pipeline.
For instance, in September 2025, Toxys’ ToxTracker assay been officially approved by the OECD for inclusion in its Test Guideline Programme. This stem cell-based in vitro assay detects genotoxicity and cellular stress responses, offering pharmaceutical companies a validated, animal-free method for early-stage drug safety screening. The move strengthens global adoption of ethical toxicity testing practices.
To learn more about this report, Download Free Sample
North America, holding 47.30% share in 2025, is projected to dominate the in vitro toxicity testing market, driven by strong pharmaceutical and biotech sectors, stringent regulatory frameworks, and ethical shifts away from animal testing. Advanced technologies like high-throughput screening and toxicogenomics are widely adopted, supported by government initiatives and research funding. The region's leadership in drug development accelerates market growth and innovation.
For instance, in September 2025, BioIVT hosted and participated in major ADME-focused conferences this fall, including Discovery on Target in Boston and AAPS PharmSci 360 in Orlando. The company aims to showcase its latest advancements in absorption and metabolism research, reinforcing its role in supporting in vitro toxicity testing and drug development across North America.
Asia Pacific is anticipated to be the fastest growing region, due to rising pharmaceutical R&D, stricter regulatory standards, and growing demand for ethical, non-animal testing methods. Rapid biotech expansion in countries like China, India, and Japan, along with cost-effective lab infrastructure and skilled workforce, further fuels adoption of cell-based assays and alternative toxicology technologies.
For instance, in April 2025, the Chinese University of Hong Kong launched Asia’s first organoid biobank for bladder cancer, advancing personalized medicine in the region. Using patient-derived stem cells, the biobank enables 3D cell culture models for drug screening and treatment planning. This initiative supports precision oncology and enhances research capabilities across Hong Kong and the Asia-Pacific.
China’s in vitro toxicity testing market is surging due to stricter cosmetic and pharmaceutical regulations, growing biotech investments, and a national push to reduce animal testing. The adoption of OECD-aligned standards and advanced cell-based assays supports safer, ethical product development, making China a key driver of innovation and demand in Asia-Pacific.
For instance, in October 2025, China’s National Institutes for Food and Drug Control (NIFDC) has released six draft cosmetic standards for public consultation, including in vitro mammalian cell chromosomal aberration and bacterial reverse mutation test methods. These updates align with global non-animal testing trends and signal China’s growing commitment to standardized, science-based cosmetic safety assessments.
Japan’s in vitro toxicity testing market is growing due to strict regulatory standards, ethical concerns over animal testing, and advanced biomedical research infrastructure. The country’s investment in tissue engineering, cell-based assays, and OECD-aligned methods supports safer, non-animal testing for cosmetics and pharmaceuticals, driving demand across academia, industry, and regulatory sectors.
For instance, in December 2024, Japan Tissue Engineering Co., Ltd. (J-TEC) partnered with Shiven Biotech to distribute LabCyte cultured human tissue products in India. These models, including EPI-MODEL and CORNEA-MODEL, offer ethical alternatives to animal testing for cosmetics and medical products. The collaboration supports India’s growing demand for in vitro toxicity testing and aligns with global non-animal testing trends.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 10.04 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 13.5% | 2032 Value Projection: | USD 24.36 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Bio-Rad Laboratories, Inc., Cyprotex, Covance Inc., LifeNet Health LifeSciences, Creative Bioarray, Charles River Laboratories, Intertek Group plc, LGC Limited, SGS Société Générale de Surveillance SA, Preferred Cell Systems, Microbac Laboratories, Inc., Eurofins Discovery, Creative Biolabs, LAUS GmbH, and Vimta Labs Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The in vitro toxicity testing market growth is being accelerated by increasing inorganic activities among market players and research institutes. Strategic collaborations, mergers, acquisitions, and licensing agreements are enabling companies to expand their technological capabilities and geographic reach. Research institutes are partnering with biotech firms to commercialize advanced cell-based assays and non-animal testing platforms. These inorganic moves foster innovation, regulatory compliance, and faster product development cycles. As demand for ethical and efficient toxicity testing rises, such alliances are crucial for scaling operations, meeting global standards, and driving competitive advantage in the evolving in vitro toxicology landscape.
For instance, in March 2023, the Institute for In Vitro Sciences (IIVS), U.S. and Toxys, a biotechnology company, entered into a license agreement that allows IIVS to offer the ToxTracker assay. ToxTracker is an in vitro assay that allows identification of the genotoxic and potentially carcinogenic properties of novel and existing drugs, agrochemicals, cosmetics and other substances without the use of animal testing.
Organ-on-chip and 3D cell culture models are revolutionizing in vitro toxicity testing by replicating human organ physiology more accurately than traditional 2D cell cultures. These advanced systems mimic the structural and functional complexity of tissues, enabling more predictive assessments of drug toxicity and efficacy. Organ-on-chip platforms integrate microfluidics to simulate blood flow and mechanical forces, while 3D organoids recreate multicellular environments. These innovations are particularly valuable for studying systemic toxicity, neurotoxicity, and endocrine disruption, where traditional models fall short. As pharmaceutical and biotech industries seek more human-relevant data, these technologies are becoming essential tools in preclinical safety evaluation and are expected to significantly influence the In Vitro Toxicity Testing Market forecast.
The global shift away from animal testing, especially in cosmetics, is driving strong demand for in vitro toxicity testing methods. Regulatory bans in regions like the European Union and India have mandated the use of alternative, non-animal approaches for safety assessments. In vitro assays, including skin and eye irritation tests using reconstructed human tissues, offer ethical and scientifically robust solutions. Additionally, chemical manufacturers are adopting these methods to comply with REACH and other international safety regulations. This trend is fostering innovation in assay development, encouraging investment in validated platforms, and expanding the market for cell-based and high-throughput toxicity testing technologies, as highlighted in recent In Vitro Toxicity Testing Market research.
The global in‑vitro toxicity testing market value is undergoing a strategic transformation, moving beyond traditional 2D cell‑culture assays toward advanced platforms such as 3D models, organ‑on‑chip systems, and multi‑omics approaches. These emerging technologies, growing at double‑digit rates, are enabling more predictive toxicology and offering a competitive advantage to early adopters. Legacy assay providers risk margin pressure, while firms integrating AI-driven analytics and high-throughput systems can command premium pricing.
Regulatory alignment has shifted from a barrier to a differentiator. Quantitative in vitro to in vivo extrapolation (QIVIVE) and OECD test-guideline validation have narrowed translational gaps, allowing regulatory bodies to increasingly endorse non-animal methods. CROs demonstrating regulatory-grade platforms are better positioned, whereas others may face commoditisation.
Market bifurcation is evident: high-value mechanistic platforms will command premium pricing, while routine assays face pricing pressure. Capital-intensive technology adoption, validation gaps, and extrapolation uncertainties remain challenges. Strategic differentiation, regulatory validation, geographic expansion, and integrated analytics will define success in this increasingly complex market.
Definition: In Vitro Toxicity Testing is the scientific evaluation of the hazardous effects of chemical compounds on cultured bacteria or mammalian cells. In vitro testing techniques are mainly used in the early stages of the development of potentially useful new substances such as therapeutic drugs, agricultural chemicals, and food additives, to identify potentially hazardous chemicals and/or to confirm the lack of certain toxic properties.
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients