In-vitro diagnostics market is estimated to be valued at USD 126.73 Bn in 2025 and is expected to reach USD 197.06 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 6.5% from 2025 to 2032.
To learn more about this report, Download Free Sample
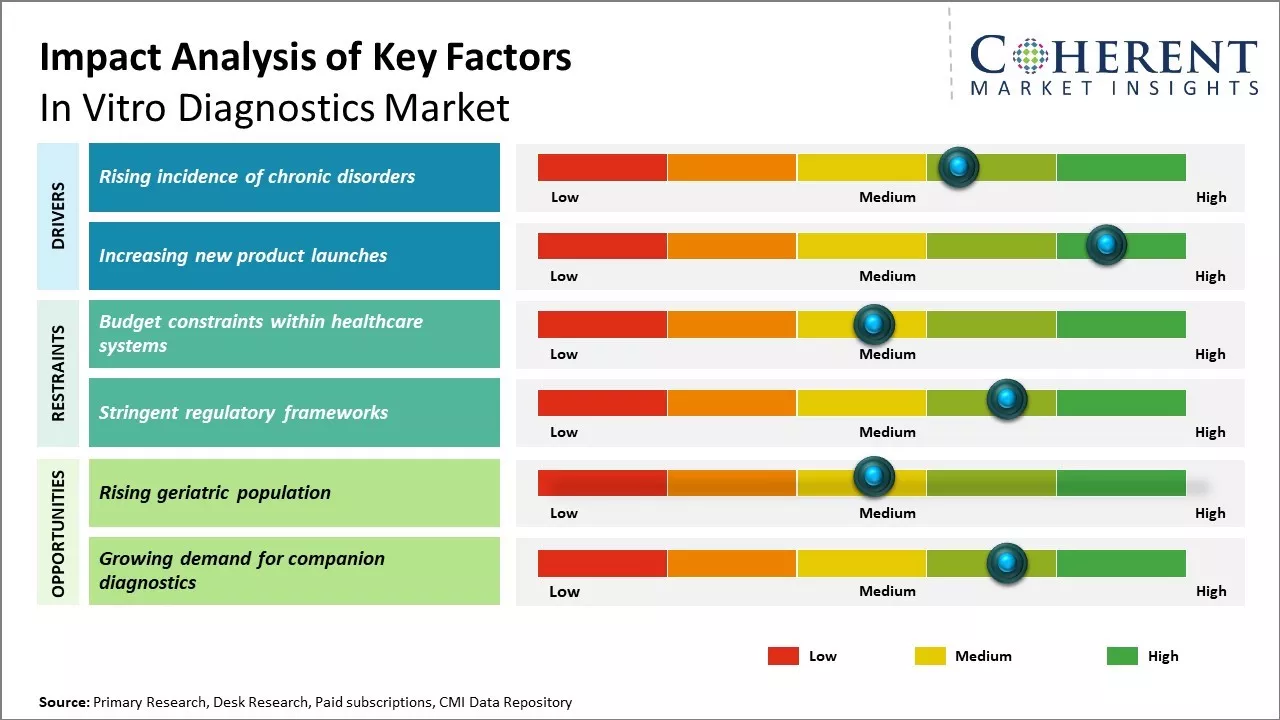
The market is expected to witness positive growth over the forecast period owing to rising geriatric population globally and increasing adoption of point-of-care testing. Demand for in-vitro diagnostic tests is increasing due to rising prevalence of chronic and infectious diseases. Additional factors such as technological advancements in IVF technologies, growing awareness regarding early disease diagnosis, growing demand for personalized medicine will further drive the in vitro diagnostics market growth during this period.
|
Current Event |
Description and its Impact |
|
AI and Digital Health Revolution in Diagnostics |
|
|
Aging Global Population and Chronic Disease Burden |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The changing lifestyle and dietary patterns prevalent worldwide have led to increase in chronic medical ailments. Conditions like cardiovascular disease, diabetes, cancer are no longer perceived as diseases of affluence but have affected people across socioeconomic classes. Long-term exposure to environmental contaminants and rising levels of stress have further contributed to non-communicable diseases gaining epidemic proportions in many developed and developing regions.
Unlike acute illnesses, these long-lasting health issues require constant clinical monitoring and therapy adjustments based on test results. Advanced diagnostic assays aid in prognostication and disease management by identifying biological markers, characterizing the extent or stage of the disease, detecting treatment effectiveness or resistance. These empower physicians to personalize treatment approaches.
Increasing new product launches by key market players is expected to drive the global in-vitro diagnostics market growth. For instance, in June 2022, Streck, a manufacturer of laboratory products, announced the commercialization of the Streck Viral Extraction Kit with partner Ceres Nanosciences, a life sciences company. Streck has developed a virus extraction kit using Nanotrap technology to reduce manual effort and simplify the workflow for isolating SARS-CoV-2 and Influenza A from clinical samples.
Product segment is sub-segmented into reagents & kits and instruments. Reagents & kits segment is anticipated to hold 68.3% of the market share in 2025. Advancements in medical care have led physicians to rely heavily on laboratory testing to better understand diseases and determine appropriate treatment plans. As diagnostic capabilities expand, more reagents and kits are needed to perform the wide range of tests available.
Being consumable products that must be replenished regularly, reagents and kits witness high and recurring demand from hospitals, clinics, and other testing facilities. Their ability to work with various automated and non-automated analyzers also makes them a more universal option for laboratories compared to proprietary instruments. Manufacturers continue adding new reagents and assays to test menus to keep up with the evolving diagnostic needs.
The demand for reagents and kits is also boosted by the trend of decentralization where diagnostic testing is being brought closer to patients through small laboratories, POC testing, and home use. This decentralized model improves access and boosts adoption of simple, cartridge-based reagents and kits that can be used outside traditional diagnostic settings, further accelerating the in vitro diagnostics market demand.
For instance, in September 2023, NeoDx Biotech Labs, a Bengaluru-based startup launched Real-time PCR-Technology-based in vitro diagnostic (IVD) kit for Ankylosing Spondylitis & empowers healthcare services to improve their testing capabilities.
Test type segment is sub-segmented into clinical chemistry, immunoassay, hematology, molecular diagnostics, microbiology, coagulation & hemostasis, urinalysis, and others. Immunoassay segment contributes the highest share of the in-vitro diagnostics market and is projected to hold 34.6% of the market share in 2025.
Immunoassays utilize the principle of antigen-antibody binding, and are highly sensitive and specific for detecting targets like proteins, antibodies, hormones, and others in patient samples. Their versatility has made immunoassays the primary technology used to diagnose HIV, hepatitis, influenza and other communicable illnesses. Rising prevalence of infectious diseases, especially in developing regions, is a key factor propelling the market growth.
Factors like lack of adequate sanitation, vector proliferation, and limited vaccination drive up infections rates. Diagnosing these diseases early and tracking epidemics have become public health priorities, contributing significantly to immunoassays demand. Other promoting factors are new assays for emerging diseases and the ability of immunoassays to provide rapid results. Automated analyzers have also boosted efficiency and throughput. Continuous technology refinements will help immunoassays maintain their leading role in infectious disease diagnostics.
For instance, in June 2025, H.U. Group Holdings Inc. and its wholly-owned subsidiary Fujirebio announced the availability of the Lumipulse G sTREM2 assay for the fully automated LUMIPULSE® G immunoassay analyzers.
Application segment is sub-segmented into infectious diseases, diabetes, oncology, cardiology, nephrology, autoimmune disorders, and others. Infectious diseases segment contributes the highest share of the in-vitro diagnostics market and is projected to hold 48.7% of the market share in 2025.
The ability to quickly and accurately diagnose infectious diseases through various IVD tests is becoming increasingly important in clinical care and public health monitoring. As newer pathogens emerge and existing ones adapt and spread into new regions, the demand for advanced diagnostic capabilities is growing steadily.
To learn more about this report, Download Free Sample
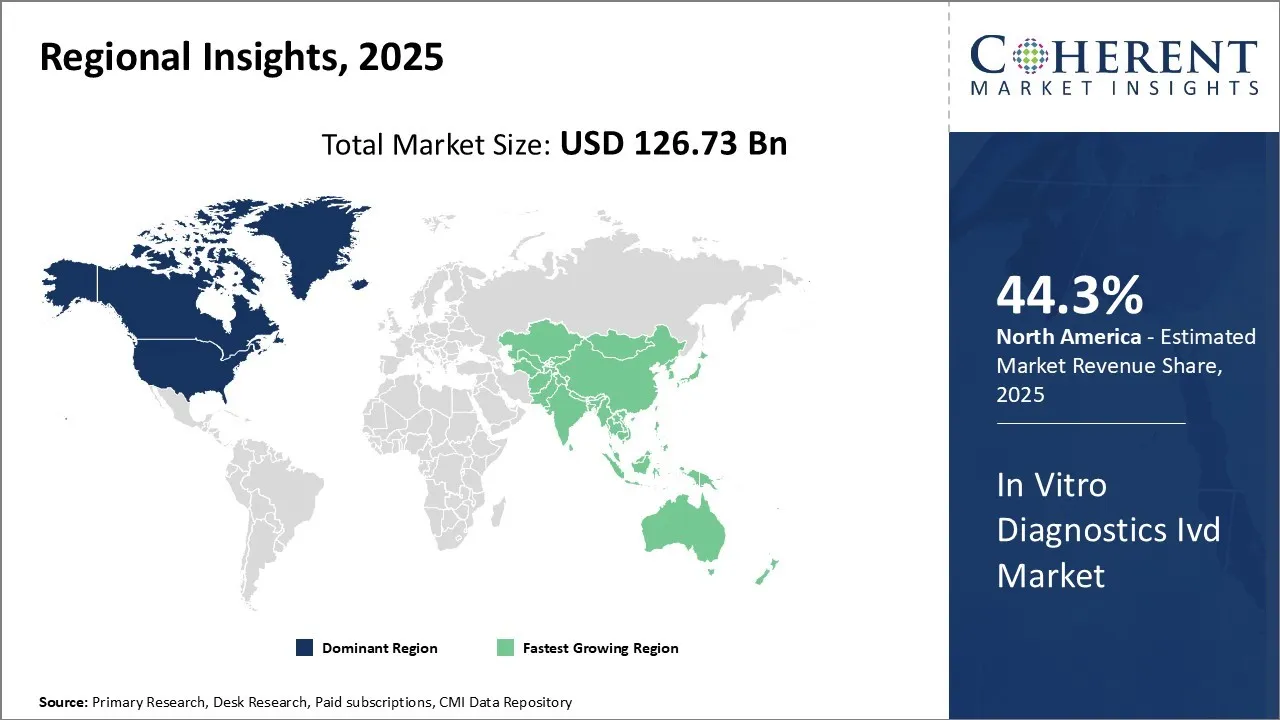
North America has established itself as the dominant region in the global in-vitro diagnostics market and is expected to hold 44.3% of the market share in 2025. The large presence of leading IVD companies such as Thermo Fisher Scientific, Abbott Laboratories, and Becton Dickinson has enabled the region to evolve considerably over the years in terms of innovation and product offerings.
With some of the most stringent regulatory guidelines enforced by authorities such as the U.S. FDA, North American companies have set the benchmark for producing high-quality diagnostics products. Most new diagnostic technologies and platforms are commercialized first in this region, allowing companies to gain large market share before expanding globally. In addition, favorable reimbursement policies for diagnostic tests make the region highly lucrative.
According to the Centers for Disease Control and Prevention (CDC), approximately 129 million individuals in the U.S. live with at least one major chronic condition, including heart disease, cancer, diabetes, obesity, or hypertension.
The Asia Pacific region, on the other hand, is emerging as the fastest growing market. Several factors are contributing to this rapid growth like improving healthcare infrastructure, rising medical tourism, and growing investments by foreign players looking to tap the opportunities in developing economies.
Country-level analyses indicate China and India will spearhead growth due to their huge patient populations and increasing healthcare expenditures. Both nations are working to reduce their dependence on imports and promote local manufacturing of IVD devices and reagents.
Several global companies have established manufacturing facilities as well as research centers in these countries. Meanwhile, other Asian economies like South Korea, Japan, Singapore, Malaysia and Thailand offer attractive business environments and act as manufacturing and export hubs to cater to the demand across Asia and other regions.
For instance, in February 2023, Redcliffe Labs introduced a molecular diagnostic test in India to detect multidrug-resistant TB (MDR-TB). This test offers faster and more accurate detection, addressing the rising need for advanced TB diagnostics and improving disease control efforts in the region.
The U.S. leads the in vitro diagnostics industry, driven by major industry players such as Roche Diagnostics, Abbott, and Danaher. Their dominance is reinforced by strong R&D investments and favorable reimbursement policies that support innovation and the adoption of advanced diagnostic technologies.
According to the American Cancer Society, over 2 million new cancer cases are projected to be diagnosed in the U.S. in 2024, excluding basal and squamous cell skin cancers and noninvasive carcinoma in situ, except for urinary bladder cases. This rising disease burden is expected to sustain the country's leadership in the market.
The U.K in vitro diagnostics market is experiencing significant growth, driven by a robust healthcare infrastructure, high disposable income, and increasing awareness of early disease detection. The rising demand for molecular diagnostics is further supported by government initiatives aimed at improving accessibility to diagnostic services and reducing NHS waiting times.
For instance, in July 2023, Virax Biolabs Group Limited, a company specializing in immune response detection and viral disease diagnostics, announced plans to launch two new research and laboratory facilities in the UK.
To learn more about this report, Download Free Sample
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 126.73 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.5% | 2032 Value Projection: | USD 197.06 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
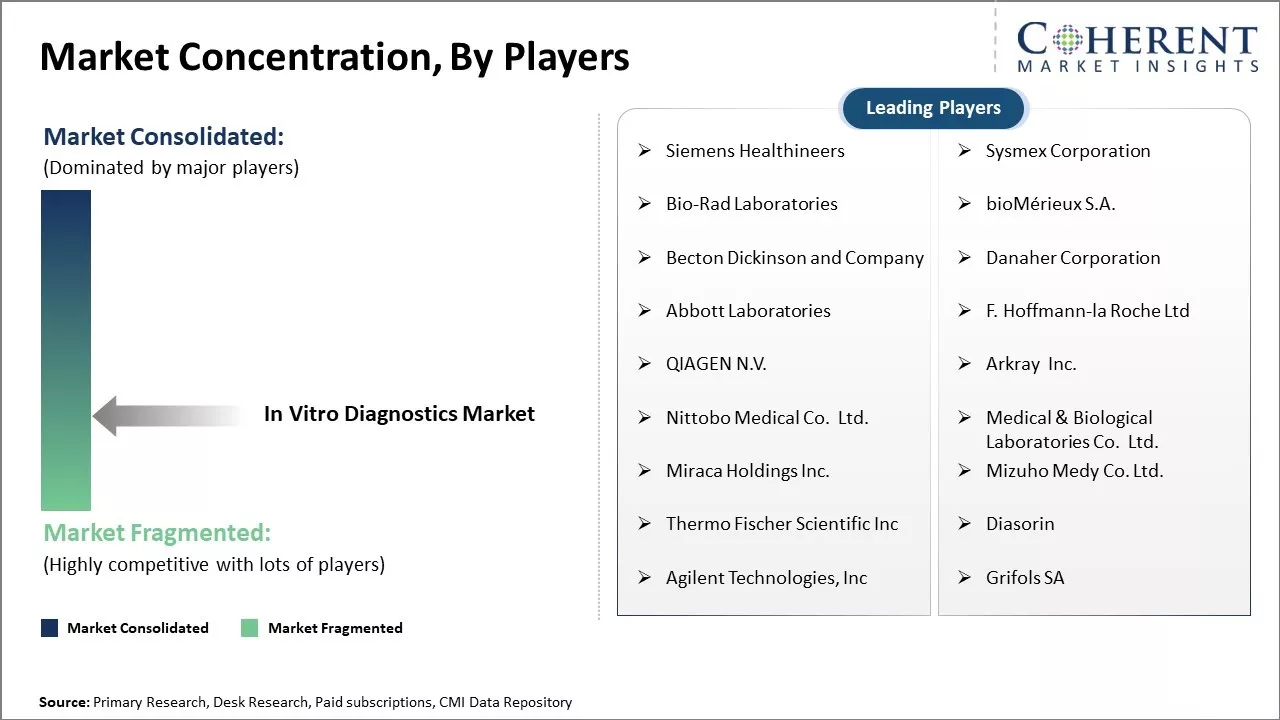
Siemens Healthineers, Sysmex Corporation, Bio-Rad Laboratories, bioMérieux S.A., Becton Dickinson and Company, Danaher Corporation, Abbott Laboratories, F. Hoffmann-la Roche Ltd, QIAGEN N.V., Arkray Inc., Nittobo Medical Co. Ltd., Medical & Biological Laboratories Co. Ltd., Miraca Holdings Inc., Mizuho Medy Co. Ltd., Thermo Fischer Scientific Inc, Diasorin, Agilent Technologies, Inc, Grifols SA |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Growing geriatric population and increasing prevalence of chronic and infectious diseases boosts demand for diagnostic testing. Telehealth and home healthcare are opening new channels for diagnostic services. Further, point-of-care testing is gathering momentum due to its convenience and quick results. Partnerships along the healthcare continuum can help in vetting new technologies and expanding access.
*Definition: In-Vitro Diagnostics Market involves products, instruments and reagents used to test specimens taken from the human body, such as blood, urine and tissue. These diagnostic tests are performed outside of a living body in controlled laboratory settings using instruments and reagents to examine various biomarkers that can help identify diseases and other medical conditions. Some common types of tests included in this market are immunoassays, clinical chemistry, molecular diagnostics, microbiology and hematology.
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients