Implant And Prosthesis Fastener Market is estimated to be valued at USD 16.11 Bn in 2025 and is expected to reach USD 23.28 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 5.4% from 2025 to 2032.
Analysts’ Views on Global Implant & Prosthesis Fastener Market:
Increased collaboration and acquisition strategies by the major key players are anticipated to fuel the expansion of the global implant & prosthesis fastener market over the forecast period, as well as rising fracture rates brought on by osteoporosis' high prevalence. For instance, in July 2022, Unlimited Tomorrow, a manufacturer that supplies individuals and clinicians with TrueLimb robotic prosthetic arm, announced a partnership with The Singularity Group AG, a U.S.-based company that offers executive educational programs, a business incubator, and business consulting services, to bring functional prosthetic limbs to those in need. Together, these organizations are launching a US$ 1 million GoFundMe initiative to create and deliver functional prosthetic limbs to 100 amputee victims of the Russian invasion of Ukraine.
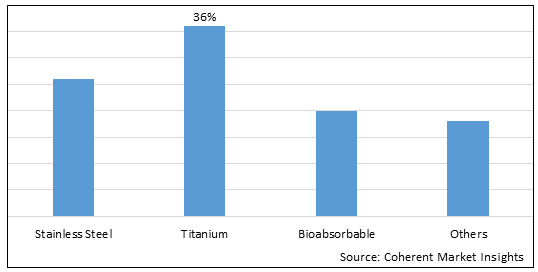
Figure 1. Global Implant & Prosthesis Fastener Market Share (%), By Type, 2025
To learn more about this report, Download Free Sample
Global Implant & Prosthesis Fastener Market– Drivers
Increasing prevalence of fractures due to high prevalence of osteoporosis
The increasing prevalence of fractures due to the high prevalence of osteoporosis is expected to drive the market growth over the forecast period. For instance, in July 2021, according to the International Osteoporosis Foundation, osteoporosis affected approximately 6.3% of men over the age of 50 and 21.2% of women over the same age range. Globally, osteoporosis causes more than 8.9 million fractures annually, resulting in an osteoporosis fracture every 3 seconds.
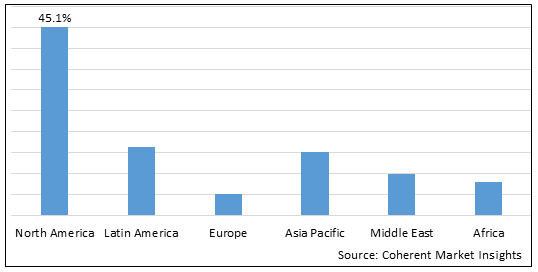
Figure 2. Global Implant & Prosthesis Fastener Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Global Implant & Prosthesis Fastener Market- Regional Analysis
Among regions, North America is estimated to hold a dominant position in the global implant & prosthesis fastener market over the forecast period owing to an increase in the prevalence of fractures due to osteoporosis and the adoption of growth strategies by the key players such as collaboration as well as acquisition. For instance, on April 6, 2025, Henry Schein, Inc., a provider of healthcare solutions to office-based dental and medical practitioners, announced that it had completed the acquisition of a majority ownership stake in Biotech Dental, a rapidly growing provider of dental implants, clear aligners, and innovative digital dental software.
Global Implant & Prosthesis Fastener Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others faced problems regarding transportation of drugs from one place to another.
However, the COVID-19 pandemic had a negative impact on the global implant & prosthesis fastener market. The spread of coronavirus is negatively impacting the global implant & prosthesis fastener market as many surgeries such as spine surgeries, dental surgeries, etc. were cancelled or postponed due to the COVID-19 pandemic. For instance, in February 2021, according to an article published in the Journal of BioMed Central, the COVID-19 pandemic delayed the implantation of definitive prosthesis for a further 3 weeks due to the patient’s acute pulmonary distress. Thus, delays in the implantation of prostheses negatively affected the global implant and prosthesis fastener market.
Implant & Prosthesis Fastener Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 16.11 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.4% | 2032 Value Projection: | USD 23.28 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Johnson & Johnson Services, Inc., OSSTEM IMPLANT CO., LTD., Stryker, Zimmer Biomet, Nobel Biocare Services AG, Institut Straumann AG, Dentsply Sirona, Smith+Nephew, Acumed LLC, OsteoMed, B. Braun SE, and Dentium USA. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Implant & Prosthesis Fastener Market- Segmentation
The global implant & prosthesis fastener market report is segmented into type, application, end user, and region.
By Type, the global implant & prosthesis fastener market is segmented into stainless steel, titanium, bioabsorbable, and others. Out of which, the titanium segment is expected to dominate the market during the forecast period, and this is due to its biocompatibility, low cost, and capability of inducing little or no deleterious effect on the surrounding tissue.
By Application, the global implant & prosthesis fastener market is segmented into dental, spinal, orthopedic, and facial. Out of which, the dental segment is expected to dominate the market during the forecast period, and this is due to the increasing prevalence of chronic diseases and other disorders in geriatric patients.
Among End User, the global implant & prosthesis fastener market is segmented into hospitals, clinics, and ambulatory surgical centers. Out of which, the hospitals segment is expected to growth in the global implant & prosthesis fastener market over the forecast period, and this trend is linked to an increase in the number of patients with musculoskeletal diseases worldwide, an increase in the number of traffic accidents that result in fractures, and hospitals' use of new and cutting-edge technologies.
Among Region, the global implant & prosthesis fastener market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa. Out of which, North America is expected to dominate the market over the forecast period owing to an increase in the prevalence of fractures due to osteoporosis and the adoption of growth strategies by the key players, such as collaboration as well acquisition.
Among all segmentation, the type segment has the highest potential due to the increasing partnership as well as acquisition activity by the key market players. For instance, in October 2020, PROTEORUSA, a wholly owned subsidiary of the France-based Proteor Group, signed an agreement with Ottobock, a company that operates in the field of orthopedic technology, to acquire a significant portion of the lower limb prosthetics portfolio of Freedom Innovations, which demonstrates Proteor’s continued commitment and investment in the worldwide prosthetic industry. Under the agreement, Proteor will substantially expand its lower limb prosthetics portfolio and acquire a range of Freedom Innovations’ flagship products, including Plié3 microprocessor knee, Kinnex and Kinterraankles, Agilix, Highlander, Dynadapt, Sierra, and Pacifica foot products.
Global Implant & Prosthesis Fastener Market- Cross Sectional Analysis
Among types, the titanium segment held a dominant position in the North America region owing to the increasing prevalence of fractures caused due to osteoporosis and adoption of growth strategies by the market key players. For instance, in March 2022, American Banknote Corporation (ABCorp), one of the longest-standing manufacturing service providers in the U.S., announced a partnership with Unlimited Tomorrow, for the production of its TrueLimb prosthetic Limbs.
Global Implant & Prosthesis Fastener Market- Key Developments
In November 2022, Zimmer Biomet, a global medical technology company, announced the U.S. Food and Drug Administration (FDA) 510(k) clearance for Persona OsseoTi Keel Tibia for cementless knee replacement. Persona OsseoTi is the latest addition to the clinically proven Persona Knee System and features a new porous version of Persona anatomic tibia with Zimmer Biomet's OsseoTi Porous Metal Technology, which uses anatomical data in combination with 3D printing technology to build a structure that directly mimics the architecture of human cancellous – or spongey – bone. This material is combined with a keeled design to deliver stable initial and biological fixation.
In July 2022, Acumed LLC., a medical device manufacturer company, announced the acquisition of ExsoMed, a medical device company providing orthopedic surgeons with innovative solutions in hand surgery. The addition of ExsoMed, whose products support an intramedullary approach to treating hand fractures, enhances Acumed’s comprehensive portfolio of upper extremity solutions for simple to complex injuries.
In October 2022, the University of Utah Bionic Engineering Lab developed "Utah Bionic Leg," the most advanced bionic leg ever created. The university has forged a new partnership with the global company in the prosthetics industry, Ottobock, to license the technology behind Utah Bionic Leg and bring it to individuals with lower-limb amputations.
In December 2020, the U.S. Food and Drug Administration approved Osseoanchored Prostheses for the Rehabilitation of Amputees (OPRA) Implant System, the first implant system marketed in the U.S. for adults who have transfemoral—or above-the-knee—amputations and who have or are anticipated to have rehabilitation problems with, or cannot use, a conventional socket prosthesis.
Global Implant & Prosthesis Fastener Market- Key Trends
Awareness regarding prosthesis
Awareness regarding prosthesis is to be taken under consideration to further explain the clinical benefits of microprocessor-controlled knees. For instance, in January 2022, according to the study published by the Virginia Commonwealth University (VCU), VCU College of Health Professions’ Department of Physical Therapy received a US$ 1.97 million grant in 2021 from the U.S. Department of Defense for its four-year project titled “Exploring the Impact of Microprocessor-Controlled Knees on Prosthesis Awareness and Overall Health.” The project will ultimately implement a newly developed measure of prosthesis awareness to further explain the clinical benefits of microprocessor-controlled knees.
Global Implant & Prosthesis Fastener Market: Restraints
Infection associated with prosthesis
Infection associated with prostheses is expected to hinder market growth over the forecast period. For instance, on April 22, 2023, according to an article published in Statspearl, prosthetic joint infection varies from one center to another but typically ranges between 0.5% to 1.0% for hip and shoulder replacements and 0.5% and 2% for knee replacements. To avoid the risk of infection caused by the prosthesis, the best way is to prevent it. Prevention is controlled both by the surgeon and patient factors.
Global Implant & Prosthesis Fastener Market- Key Players
Major players operating in the global implant & prosthesis fastener market include Johnson & Johnson Services, Inc., OSSTEM IMPLANT CO., LTD., Stryker, Zimmer Biomet, Nobel Biocare Services AG, Institut Straumann AG, Dentsply Sirona, Smith+Nephew, Acumed LLC, OsteoMed, B. Braun SE, and Dentium USA.
Global Implant & Prosthesis Fastener Market – Definition
Devices which are placed inside or on the surface of the body are called medical implants. Implants which are intended to replace missing body parts such as legs or arms are called prosthetics. Implants are made from plastic, ceramic, metal or other materials. The placing of implants can be permanent or they can be removed once they are no longer needed. For example, chemotherapy ports or screws to repair broken bones can be removed when they are not needed but tents, hip implants which are intended to be permanent.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients