Immunotherapy Drugs Market is estimated to be valued at USD 185.72 Bn in 2025 and is expected to reach USD 398.16 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 11.5% from 2025 to 2032.
To learn more about this report, Download Free Sample
The Immunotherapy Drugs Market Size is expanding quickly, and several sources predict that it will increase significantly over the next coming years. The immunotherapy drugs market is witnessing positive growth trends over the years. Immune checkpoint inhibitors, which help unleash the body's own immune response against cancer, have emerged as a major category of immunotherapy. Checkpoint inhibitor drugs such as Keytruda, Opdivo and Tecentriq are widely adopted drugs. Rising incidence of cancer cases globally and increasing preference for precision medicines are key factors boosting demand for immunotherapy drugs over the forecast period.
For instance, in February 2024, A team of UF Health Cancer Center researchers have developed a first-of-its-kind compound that could open a new avenue for using immunotherapy to treat various types of cancer. The substance unleashed the body's immune system to fight cancer cells, slowing the growth of established tumors and, in some cases, eliminating them in laboratory and mouse tests in skin and colorectal cancer models.
|
Event |
Description and Impact |
|
Surge in Cancer Cases in Europe |
|
|
Growing Acceptance of Monoclonal Biosimilars |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Artificial Intelligence (AI) is transforming the immunotherapy drugs market by accelerating drug discovery, optimizing clinical trials, and enhancing personalized treatment approaches. AI tools help analyze vast genomic and biomarker data, expediting the identification of new drug targets and predicting patient responses to immunotherapies.
For example, the European Union has funded initiatives such as the DREAMS project, which uses AI for target identification and drug repurposing, and the OPTIMA platform, which leverages AI to generate real-world oncology evidence for more informed clinical decisions. Technologies like digital pathology integrated with AI (e.g., Histotype Px) are enabling more precise patient stratification for colorectal cancer, directly improving therapy outcomes.
Therapy Type segment is sub-segmented into immune checkpoint inhibitors, monoclonal antibodies, vaccines, adoptive cell therapies, immune system modulators, and oncolytic virus therapy. Monoclonal antibodies segment is estimated to hold 28.9% share of the market in 2025. Monoclonal antibodies are engineered versions of human antibodies that can be designed to precisely target specific proteins on tumor or diseased cells, allowing them to eliminate those cells with minimal off-target effects.
This targeted precision has made them increasingly popular for treating various cancer types as well as autoimmune diseases. Compared to other immunotherapy drugs, monoclonal antibodies offer an ability to pinpoint disease markers and disrupt their signaling more selectively. The market has seen a rapid uptake of several monoclonal antibody drugs in recent years for treating cancers like breast and lung cancer.
Their precision also makes them well-suited for combining with other therapies like chemotherapy to achieve synergistic effects. Advancements in antibody engineering are further improving their precision and efficacy. Overall, monoclonal antibodies' mechanism of delivering targeted therapy with minimal side effects has positioned them as the leading therapy type segment.
Route of Administration segment is sub-segmented into intravenous (IV), oral, intramuscular, subcutaneous, and others. Oral segment is anticipated to hold 47.7% of the market share in 2025. Patients generally prefer oral drugs over other forms of administration as they are less invasive and can be self-administered without any medical assistance. This makes the treatment process far more convenient for patients as there is no need for hospital visits or medical supervision every time a dosage is required.
The oral drugs also provide improved treatment compliance as patients find it simpler to take pills along with their regular diet rather than regular and painful injections. The non-invasive nature of oral drugs also makes them more reassuring for patients. This significantly improves treatment outcomes as patients tend to continue their prescribed dosage schedules for longer durations without any apprehensions.
The psychological advantage of oral drugs is a big factor that contributes to their heightened preference over more interventional forms of drugs administration. Their easy availability without any prescriptions also improves accessibility and flexibility of treatment.
Indication segment includes cancer, autoimmune diseases, and others. Cancer segment is anticipated to hold 85.2% of the market share in 2025. Cancer remains one of the leading causes of mortality worldwide with over 10 million new cases diagnosed annually. The growing cancer burden has prompted heightened efforts into developing innovative immunotherapies.
Several checkpoint inhibitors and other novel modalities have been approved specifically for treating various cancer types in recent years. With improving diagnosis rates and growing focus on quality of life, cancer patients are increasingly being prescribed immunotherapy drugs. The availability of complimentary diagnostic tests has also enabled patient selection and improved outcomes.
To learn more about this report, Download Free Sample
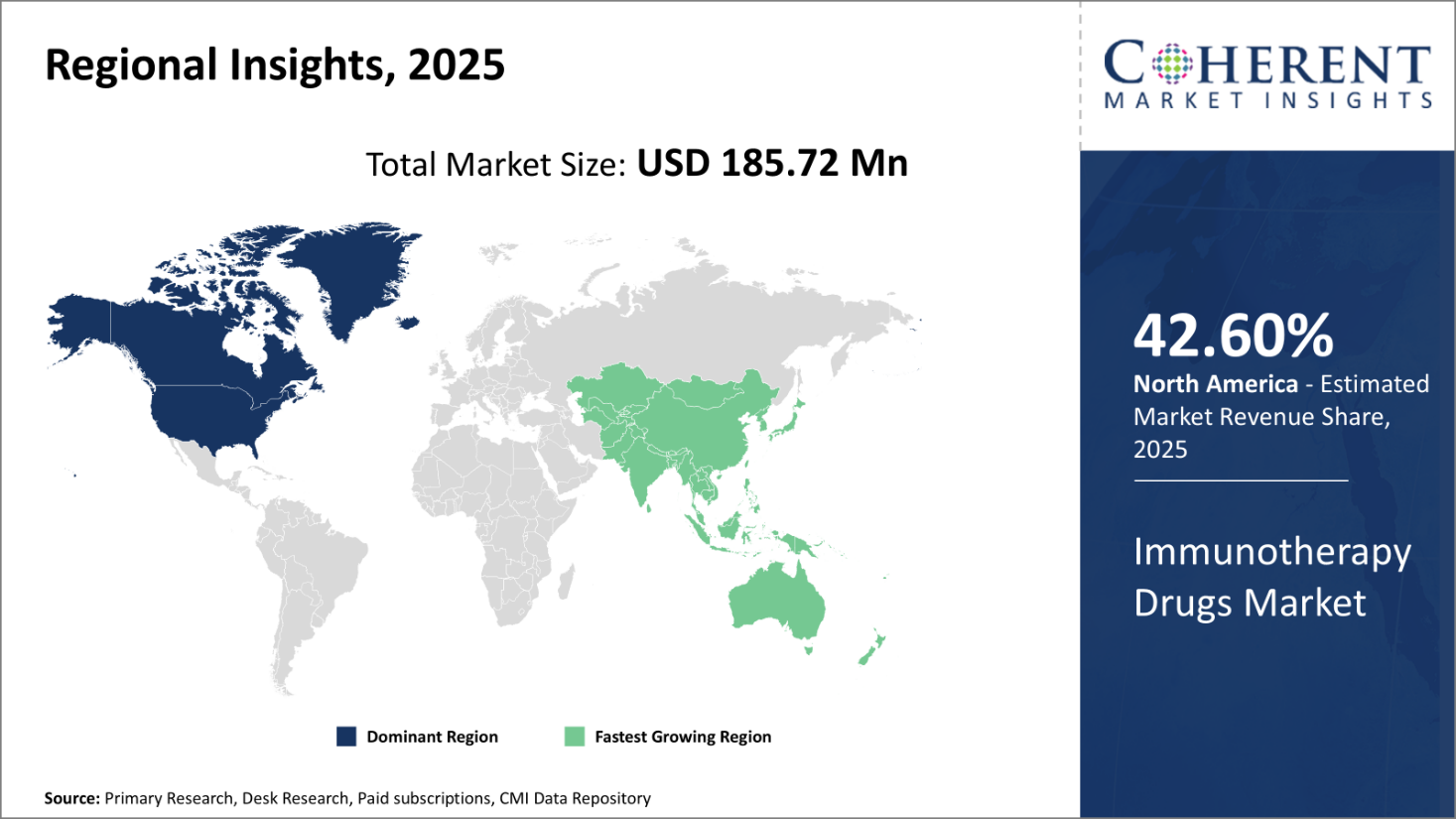
North America has established itself as the dominant region in the global immunotherapy drugs market trends and is projected to hold 42.60% of the market share in 2025. With major pharmaceutical companies headquartered in the U.S. and Canada, the region has a strong industry presence and is at the forefront of research and development of novel immunotherapies.
Several immunotherapies that have been approved in recent years were first developed by companies based in North America. This early mover advantage has helped the region gain significant market share. In addition, factors such as higher healthcare expenditure per capita, availability of favorable reimbursement policies, and presence of sophisticated healthcare infrastructure have supported adoption of high-priced immunotherapies.
North American payers have shown more willingness to pay for immunotherapies compared to other regions due to better affordability. This pricing flexibility provides an important revenue stream for immunotherapy drug manufacturers.
The Asia Pacific region, on the other hand, is witnessing the fastest growth and emerging as an important market globally. Rising healthcare expenditure, growing middle-class population, increasing focus on quality healthcare services are driving the overall oncology market in Asia Pacific, in turn aiding immunotherapy drug adoption. Countries like India, China, Japan, South Korea and Australia have become key markets within the region.
Increased use of immunotherapy medications over conventional therapies, increased demand for monoclonal biosimilars, and accelerated regulatory approvals in key European nations are driving this expansion. Immunotherapy drugs vs chemotherapy is a key consideration in current clinical practice, as the shift towards innovative treatments becomes more pronounced. Lung, liver, colon, stomach, and breast cancers are the most prevalent diseases causing this market boom; these conditions collectively cause a considerable number of fatalities in the area.
Monoclonal antibodies are the dominant segment, though cancer vaccines are emerging as the fastest-growing category in the market. The U.S. market’s leadership is also supported by widespread adoption of innovative therapies such as immune checkpoint inhibitors and CAR-T cell therapies, robust reimbursement frameworks, and the presence of leading biopharmaceutical companies.
The market for immunotherapy medications in India is growing quickly thanks to factors like rising cancer rates, increased healthcare awareness, and rising investments from both domestic and foreign pharmaceutical companies. India stands out in the Asia-Pacific area for its rapidly increasing acceptance of immunotherapy, especially for oncology.
Chinese authorities are supporting the market through accelerated regulatory approvals, substantial funding for local R&D, and efforts to foster a domestic pharmaceutical industry capable of competing internationally. The rapid uptake of checkpoint inhibitors, CAR-T therapies, and monoclonal antibody drugs is facilitated by both public and private sector investments in healthcare.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 185.72 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 11.5% | 2032 Value Projection: | USD 398.16 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
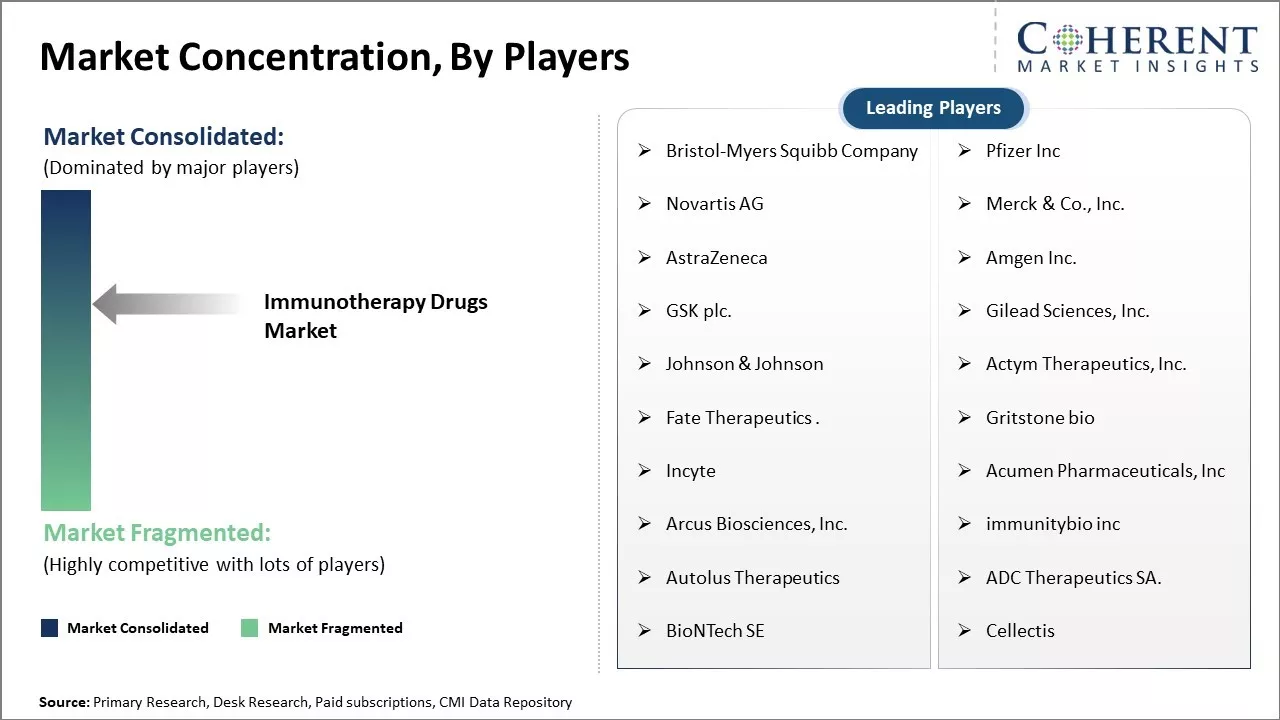
Bristol-Myers Squibb Company, Pfizer Inc, Novartis AG, Merck & Co., Inc., AstraZeneca, Amgen Inc., GSK plc., Gilead Sciences, Inc., Johnson & Johnson, Actym Therapeutics, Inc., Fate Therapeutics ., Gritstone bio, Incyte, Acumen Pharmaceuticals, Inc, Arcus Biosciences, Inc., immunitybio inc, Autolus Therapeutics, ADC Therapeutics SA., BioNTech SE, Cellectis |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
To learn more about this report, Download Free Sample
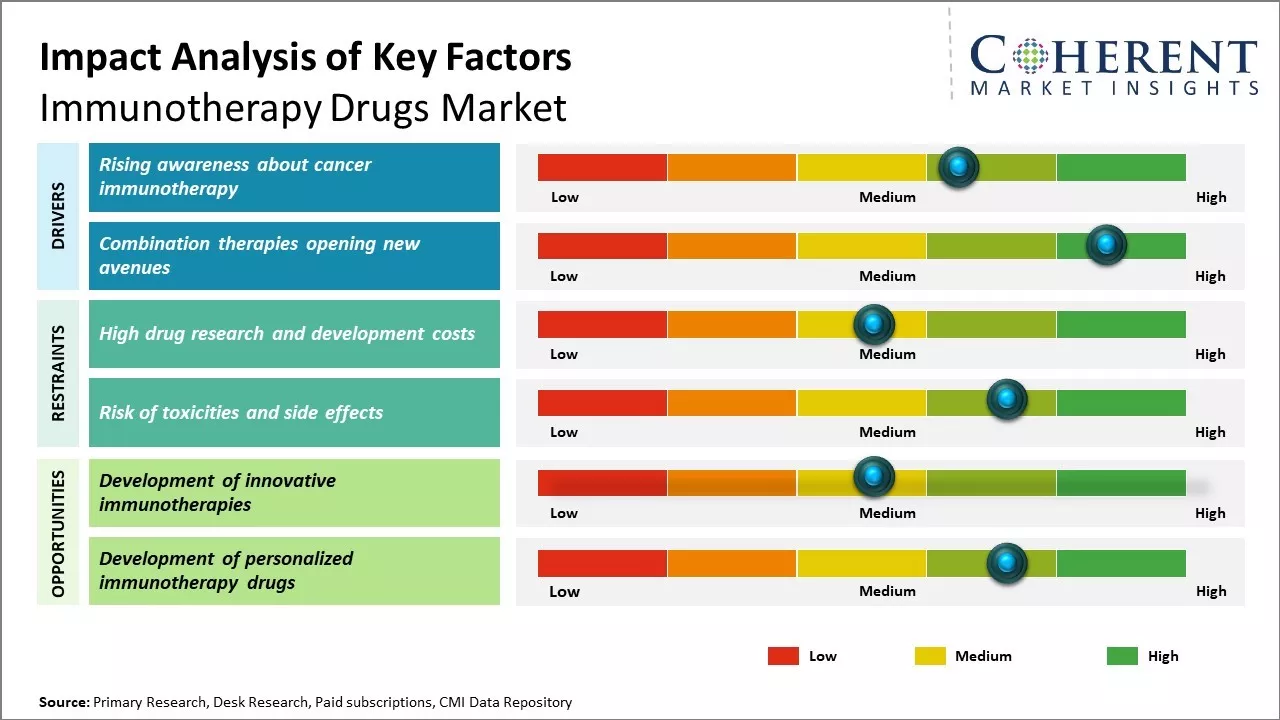
With increasing incidence of various types of cancer worldwide, there is rising awareness among public as well as medical professionals about innovative treatment options. Cancer immunotherapy is gaining prominence as it utilizes body's own immune system to fight cancer in a novel way.
Various advocacy groups are educating people about this option through awareness campaigns. These are highlighting how immunotherapy helps activate patient's own T cells to identify and destroy cancer cells in a targeted manner. This avoids non-specific killing of normal cells like other treatment options.
The success stories showcased by advocacy groups and patients who benefited from immunotherapy are helping change perceptions. Where earlier surgery or chemo was the default option, now patients ask doctors about immunotherapy upfront. Medical conferences are dedicating special sessions to share research advances and real-world experiences with immunotherapy.
Another important driver is the encouraging results seen with combining different immunotherapy drugs or combining immunotherapy with other treatment modalities. Early successes with checkpoint inhibitors led to focus on their potential to work even better when paired together or added to conventional therapies. Ongoing research is exploring diverse combination regimens tailored to individual cancer types and stages of disease. Some combinations show response rates much higher than any drug alone. These are also helping widen the pool of patients who can benefit.
For instance, combining a PD-1 inhibitor with CTLA-4 antibody is giving durable responses in melanoma, lung and other cancers. Adding these to chemotherapy prior to surgery in early breast cancer patients improves pathologic response rates. Similarly, providing immunotherapy before or after radiation helps recruit immune cells to target radiation boosted tumor cells or residuals. The scope is also extending to accelerator drugs that can enhance effects of PD-1/PDL-1 inhibitors when given together.
The market for immunotherapy medications is expanding rapidly and robustly because to the rising prevalence of chronic illnesses like diabetes and cancer worldwide, improvements in monoclonal antibodies, and large investments in science and personalized medicine. The quick uptake of personalized therapeutics is one of the major trends.
Personalized treatments that enhance patient outcomes and reduce adverse effects are made possible by genetic and biomarker research, which is frequently supported by artificial intelligence. Recent innovations in immunotherapy drugs are a key result of these advancements.
T-cell and CAR-T cell therapies are the fastest-rising segments, transforming the treatment of aggressive lymphomas and relapsed cancers, while monoclonal antibodies and immune checkpoint inhibitors have established themselves as core therapeutic categories due to their clinical effectiveness and growing indications.
The drugs have shown promising results against several cancer types and their adoption is increasing. As more clinical evidence from combination regimens emerge, response rates are expected to improve. Newer immunotherapies continue to be tested against different cancer indications providing scope for label expansions. The approaches can also potentially be adapted for non-oncology diseases thereby, creating lucrative market growth opportunities over the forecasted period.
*Definition: Immunotherapy drugs are a category of medical treatments that use a person's own immune system to fight diseases, most notably cancer. These drugs work by either stimulating or enhancing the body’s natural defenses to recognize, target, and destroy cancer cells or by providing the immune system with added components, such as laboratory-made proteins like monoclonal antibodies, to improve its ability to combat the disease.
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients