Immune checkpoint inhibitor products come under biologic therapeutic products. Biologic manufacturers require similar kind of approvals such as drugs from the U.S. Food & Drug Administration prior to bringing the product into market. However, unlike drugs, biologics require Biologics License Applications (BLA) to be filed with the Center for Biologics Evaluation and Research (CBER). Initiatives undertaken by the governments include funding is expected to drive growth of the immune checkpoint inhibitors market. For instance, in 2017, National Institutes of Health partnered with 11 leading biopharmaceutical companies such as AbbVie and Amgen, Inc. to accelerate development of new immunotherapies.
The global immune checkpoint inhibitors market is estimated to be valued at US$ 1,444.7 million in 2022 and is expected to exhibit a CAGR of 13.2% during the forecast period (2022-2030).
Figure 1. Global Immune Checkpoint Inhibitors Market Share (%), by Distribution Channel, 2022
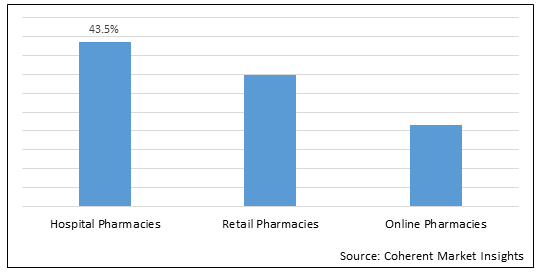
To learn more about this report, Download Free Sample
Introduction of novel immune checkpoint inhibitors with less side effects is driving global immune checkpoint inhibitors market growth
Cancer treatment has become more refined with improved treatment success rate due to improved therapeutic outcomes. This has been possible due to better understanding of the disease pathophysiology, functioning of the tumor cells, and effective ways to tackle with the same. Immune checkpoint inhibitors exhibit lesser side effects as compared to conventional cancer therapies such as chemotherapy, radiation therapy, and others. Manufactures are developing immune checkpoint inhibitors, which are tailored to attack or block particular targets. For instance, January 2018, plimumab, a human monoclonal antibody, which blocks cytotoxic T-lymphocyte-associated antigen-4 is available. Furthermore, agents focusing on specific immune regulatory checkpoints programmed death-1 (PD-1) and programmed death ligand-1 and 2 (PD-L1) such as Nivolumab, Atezolimumab, and Pembrolizumab are being used for cell lung cancer. Moreover, in April 2017, the U.S. Food and Drug Administration (FDA) granted accelerated approval to immunotherapy product- TECENTRIQ (atezolizumab) for the treatment of patients with locally advanced or metastatic urothelial carcinoma (mUC), who are not eligible for cisplatin chemotherapy, which is currently in the clinical phase 4.
Immune Checkpoint Inhibitors Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 1,444.7 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 13.2% | 2030 Value Projection: | US$ 3,902.9 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Bristol-Myers Squibb Company, Merck & Co., Inc., F. Hoffmann-La Roche AG, AstraZeneca Plc., Novartis International AG, ImmunOs Therapeutics AG, Immutep Ltd., NewLink Genetics Corporation, Ono Pharmaceutical Co., Ltd., and Pfizer, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 2. Global Immune Checkpoint Inhibitors Market Share (%), by Region, 2022
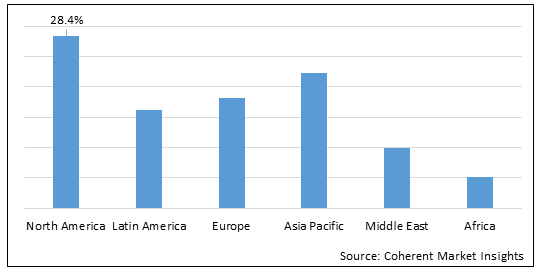
To learn more about this report, Download Free Sample
Increasing approval of the Immune Checkpoint Inhibitors by the regulatory bodies is expected to drive the global immune checkpoint market
Increasing approval of the Immune Checkpoint Inhibitors by the regulatory bodies is expected to drive the global immune checkpoint market over the forecast period. For instance, in August 2021, GSK plc., Pharmaceutical and Biotechnology Company, the U.S. Food and Drug Administration (FDA) approved GlaxoSmithKline’s PD-1 checkpoint inhibitor Jemperli (dostarlimab) for adults with mismatch repair-deficient (dMMR) recurrent or advanced endometrial cancer. Jemperli is an anti-PD-1 antibody that binds to the PD-1 receptor and blocks its interaction with the PD-1 ligands PD-L1 and PD-L2. The drug was discovered by AnaptysBio and licensed to Tesaro in March 2014. GSK completed its acquisition of Tesaro in January 2019.
Global Immune Checkpoint Inhibitors Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization had declared it a public health emergency on January 30, 2020.
COVID-19 can affect the economy in three main ways: by directly affecting production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, U.A.E., Egypt, and others, are facing problems with regards to the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a negative impact on the global immune checkpoint inhibitors market, owing to decline in number of cancer patients visits in hospitals and clinics for immune checkpoint inhibitor therapy (ICI) which lead to decline in the demand of Immune checkpoint inhibitors products. For instance, in May 2021, according to the data published by the National Center for Biotechnology Information, in response to the pandemic, the Government of India instituted a series of nationwide lockdowns that began on March 24, 2020, with severe restrictions imposed on inter-state and intra-state travel. Some cancer centres were partially or completely converted to COVID-19 treatment facilities. Data from cancer centres across the world have shown that the provision of oncology services has been considerably reduced during the COVID pandemic. Between March 1 and May 31, 2020, a substantial decrease in patient numbers was observed across all oncology services compared with the same period in 2019. The reduction in the number of patients receiving radiotherapy and palliative care were less marked than for the other services. For the period April to May 2020, the overall reduction in patient numbers across all oncology services was even more marked when compared with the same period in the previous year, especially for new patient registrations, total outpatient visits, and surgeries, which reduced by more than 60%. The percentage reduction in the number of patients accessing oncology services was higher in tier 1 cities than in tier 3 cities, with 50–75% reductions observed in almost all services provided in cancer centres in tier 1 cities between April 1 and May 31, 2020. The reductions in patient numbers were larger during April 1 to May 31, 2020 versus 2019, than during March 1 to May 31, 2020 compared with 2019.
Global Immune Checkpoint Inhibitors Market: Key Developments
In April 06 2022, the Food and Drug Administration (FDA) had approved a combination of two immunotherapy drugs for the treatment of some people with advanced melanoma. The combination consists of relatlimab and nivolumab (Opdivo) and will be marketed under the name Opdualag. Both drugs are immune checkpoint inhibitors, which target proteins called checkpoints that help stop the immune system from mounting a strong response against cancer cells. Relatlimab blocks a protein on immune cells called LAG-3, while nivolumab blocks a different protein on immune cells called PD-1. By blocking these proteins, these drugs can unleash an immune response against cancer cells. Relatlimab is the first FDA-approved drug to block the activity of LAG-3.
Global Immune Checkpoint Inhibitors Market: Restraint
The major factors that hinder growth of the global immune checkpoint inhibitors market include high cost of immuno-oncology therapies, which is unaffordable to low and middle income population. For instance, in January 2018, Keytruda (Pembrolizumab), a monoclonal antibody for the treatment of various types of cancer cost around US$ 2,250 for a vial of 50 mg.
Key Players
Major players operating in the global immune checkpoint inhibitors market include Bristol-Myers Squibb Company, Merck & Co., Inc., F. Hoffmann-La Roche AG, AstraZeneca Plc., Novartis International AG, ImmunOs Therapeutics AG, Immutep Ltd., NewLink Genetics Corporation, Ono Pharmaceutical Co., Ltd., and Pfizer, Inc.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients