Immune Check Activators Market Size and Forecast – 2026 – 2033
The global immune check activators market is estimated to be valued at USD 4.9 billion in 2026 and is expected to reach USD 12.8 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 14.6% from 2026 to 2033.
Global Immune Check Activators Market Overview
Immune Check Activators Market products primarily include therapies designed to stimulate the immune system to fight diseases, especially cancer. Key products are immune checkpoint inhibitors such as PD-1, PD-L1, and CTLA-4 inhibitors, which enhance T-cell responses. Cancer vaccines are also significant, helping the immune system recognize tumor-specific antigens. Cytokines and interleukins are used to boost immune signaling and activation. Adjuvants improve immune response strength and durability when combined with vaccines or therapies. Additionally, monoclonal antibodies play a major role by targeting immune pathways to enhance antitumor activity and improve treatment outcomes across multiple indications.
Key Takeaways
PD-1 agonists dominate the activation mechanism segment due to proven clinical efficacy and a robust development pipeline.
Oncology applications account for the largest industry share as immunotherapies become standard first-line treatments worldwide.
North America holds the largest market share, supported by advanced healthcare infrastructure and strong R&D investments.
Asia Pacific is the fastest-growing region, driven by increasing government initiatives and expanding clinical trial activity.
Immune Check Activators Market Segmentation Analysis
To learn more about this report, Download Free Sample
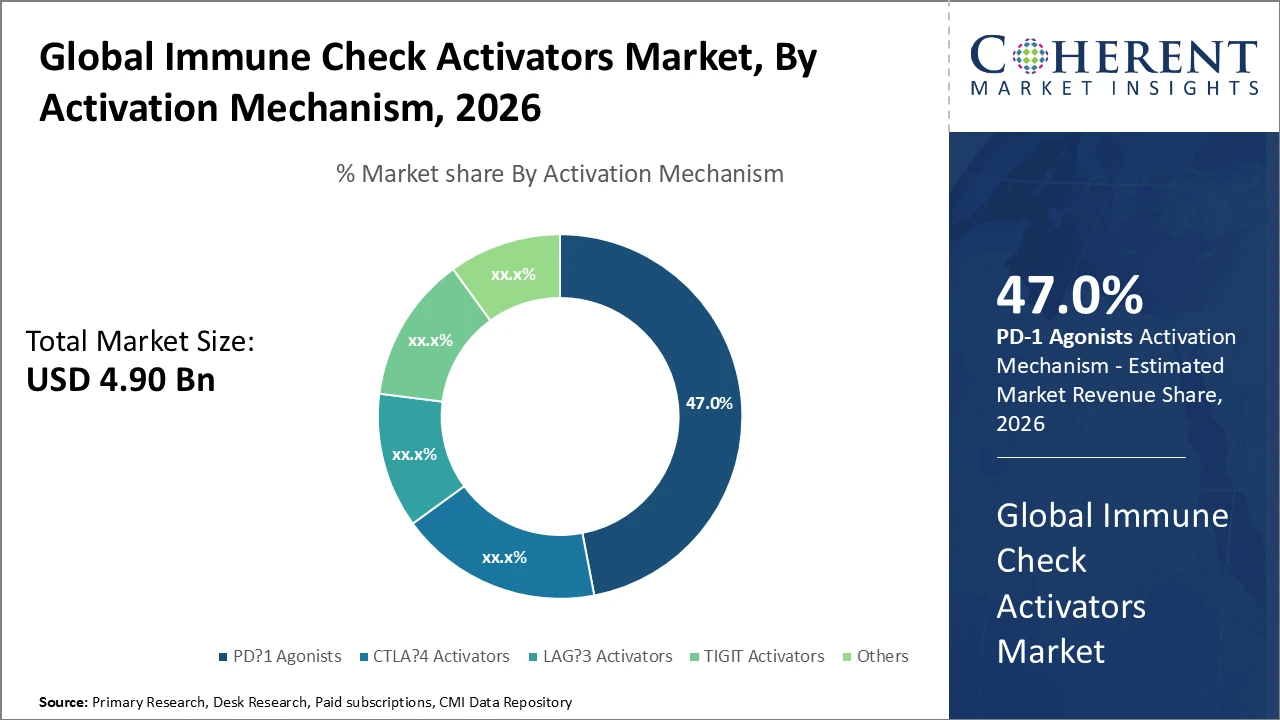
Immune Check Activators Market Insights, By Activation Mechanism
PD-1 agonists dominate the market share due to their validated clinical efficacy and widespread use across various cancer indications, supported by extensive ongoing clinical trials and multiple regulatory approvals. Their ability to effectively restore immune responses while maintaining favorable safety profiles positions them as the backbone of the market. LAG-3 activators represent the fastest-growing subsegment, driven by positive Phase II and III trial results reported in 2025 and 2026, highlighting strong synergy with PD-1 pathways. CTLA-4 activators continue to hold a solid share through their use in combination therapies, while TIGIT activators and other emerging targets are steadily expanding as research reveals new therapeutic opportunities, collectively driving future market growth.
Immune Check Activators Market Insights, By Application
Oncology dominates the market share, driven by urgent clinical needs, widespread adoption of immunotherapies, and rising global cancer incidence. Continuous advancements in cancer immunotherapy are expanding treatment indications, sustaining strong demand for checkpoint activators. Autoimmune disorders represent the fastest-growing segment, supported by the development of novel immune activators that recalibrate overactive immune responses without broad immunosuppression, enabled by recent breakthroughs in immunological pathway research. Infectious diseases and other applications remain smaller yet significant segments, focusing on immune activation to improve pathogen clearance and enhance vaccination responses. Collectively, these application areas broaden the market scope and contribute to diversified revenue growth.
Immune Check Activators Market Insights, By End-User
Hospitals dominate the market share as the primary treatment settings for immune checkpoint therapies, supported by advanced infrastructure and the integration of sophisticated immunotherapeutic protocols. The expansion of oncology centers within hospital systems and strong support for clinical trial activities further reinforce their leading position. Specialty clinics represent the fastest-growing end-user segment, driven by the increasing shift toward outpatient administration of immune therapies and the rising number of autoimmune treatments managed in specialized care environments. Research institutes remain strategically important by advancing innovation through translational research and early-stage clinical trials, while other end users, including home care settings and telemedicine services, contribute to gradual diversification of service delivery.
Immune Check Activators Market Trends
The market is shifting toward multi-targeted agents that combine immune checkpoint activation with metabolic or epigenetic pathway modulation to enhance therapeutic efficacy.
Advances in single-cell RNA sequencing and AI-driven predictive modeling in 2026 are accelerating target discovery and personalized therapy development.
Increasing focus on autoimmune disease applications is expanding the market beyond oncology, with activators designed to finely modulate immune responses.
Accelerated regulatory approvals, including fast-track and breakthrough therapy designations in the U.S. and Europe, are reducing time-to-market.
Shorter approval timelines are supporting faster clinical adoption and broader commercialization of immune check activators.
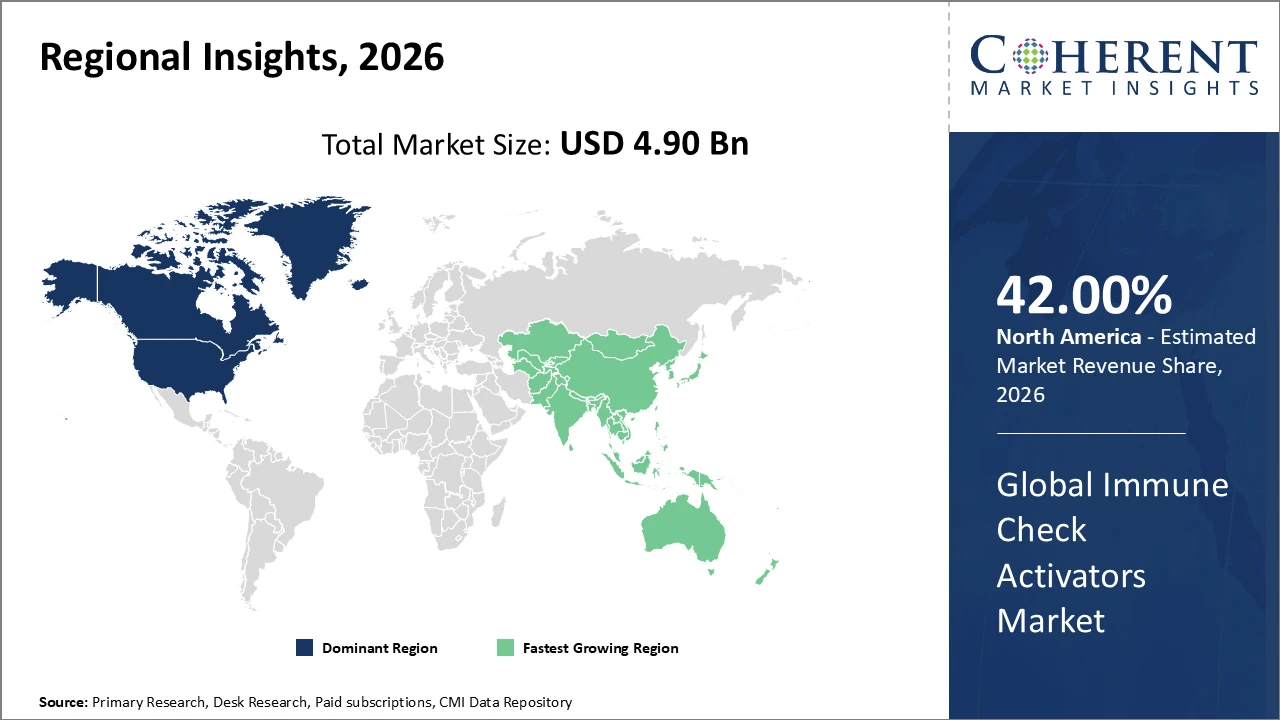
Immune Check Activators Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Immune Check Activators Market Analysis and Trends
In North America, the Immune Check Activators market is dominated by substantial R&D investments, a well-established healthcare ecosystem, and the presence of leading biotechnology and pharmaceutical companies. Advanced infrastructure, skilled medical professionals, and strong industry-academia collaborations further strengthen the region’s leadership, making North America the most influential and competitive market globally for immune activation therapies.
Asia Pacific Immune Check Activators Market Analysis and Trends
The Asia Pacific region is witnessing the fastest growth in the Immune Check Activators market, with a CAGR exceeding 17% through 2033. This growth is fueled by expanding healthcare infrastructure, increased government funding for immunotherapy research, and progressively supportive regulatory frameworks. Key countries, including China and India, are investing heavily in domestic biotechnology capabilities, driving higher market revenue and share. Additionally, the region’s rapidly evolving clinical ecosystems, combined with large patient populations, create significant opportunities for market expansion. These factors position Asia Pacific as a critical growth hub in the global immune check activators landscape.
Immune Check Activators Market Outlook for Key Countries
USA Immune Check Activators Market Analysis and Trends
The U.S. Immune Check Activators market is defined by global leadership in innovation and commercialization. By early 2026, companies based in the U.S. had launched over 60 immune checkpoint agonists and activators in clinical trials. Supportive regulatory frameworks, including the FDA’s accelerated approval pathways, enable faster market entry for innovative therapies. In 2025 alone, immunotherapy R&D investments exceeded USD 1.2 billion, reinforcing the country’s dominant position. Collaboration between major clinical centers and biotech companies facilitates real-world evidence generation, enhancing clinical adoption and market credibility. These factors collectively drive strong revenue growth and maintain the U.S.’s leadership in the global market.
Germany Immune Check Activators Market Analysis and Trends
Germany’s Immune Check Activators market is a key contributor to Europe’s overall growth, driven by advanced healthcare infrastructure, strong biopharmaceutical research, and supportive regulatory policies. The country benefits from robust clinical trial activity and collaborations between leading hospitals, research institutes, and biotech companies, enabling rapid development and adoption of immune checkpoint activators.
Analyst Opinion
The growing pipeline of immune checkpoint activators targeting PD-1, CTLA-4, and emerging targets like LAG-3 and TIGIT is a key market driver. In 2025, clinical trials for LAG-3 agonists increased by 23%, highlighting rising adoption of novel immune modulation targets.
Cost optimization in production processes has strengthened supply-side capabilities. For instance, improvements in bioreactor yields in 2024 boosted monoclonal antibody production capacity by 18%, lowering therapy costs and expanding patient access.
Demand-side trends show a shift toward combination therapies. Around 40% of ongoing trials in 2026 involve combination approaches to achieve synergistic effects and target broader indications beyond oncology, driving market growth.
Region-specific analysis indicates a 15% increase in Asia Pacific imports of immune activators in 2025, supported by expanding healthcare infrastructure and government incentives to promote local production and adoption.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 4.9 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 14.6% | 2033 Value Projection: | USD 12.8 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Merck & Co., Roche Holdings AG, AstraZeneca, Novartis AG, Pfizer Inc., Sanofi, Amgen Inc., BioNTech SE, Moderna, Inc., GlaxoSmithKline | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Immune Check Activators Market Growth Factors
The global rise in cancer incidence, particularly in developed economies, is a major driver of the Immune Check Activators market, as immunotherapy increasingly becomes a frontline treatment supported by strong efficacy data from 2025 clinical studies. Advances in biomarker identification and patient stratification have enhanced therapeutic outcomes, boosting demand for immune checkpoint activators. Additionally, increased healthcare spending and favorable reimbursement policies in North America and Europe have expanded market accessibility and adoption. Ongoing innovations in antibody engineering, cellular therapies, and multi-targeted immune activators are driving industry trends toward safer and more effective treatments. Collectively, these factors are fueling market growth, increasing revenue potential, and reinforcing the strategic importance of immune check activators in modern therapeutic landscapes.
Immune Check Activators Market Development
In April 2022, Regeneron Pharmaceuticals, Inc. announced a definitive agreement to acquire Checkmate Pharmaceuticals, Inc., a clinical-stage biopharmaceutical company developing immune-based cancer therapies. Under the all-cash transaction, Regeneron agreed to purchase Checkmate for $10.50 per share, valuing the company at approximately $250 million in equity.
Key Players
Leading Companies of the Market
Merck Co.
Roche Holding AG
AstraZeneca
Novartis AG
Pfizer Inc.
Sanofi
Amgen Inc.
BioNTech SE
Moderna, Inc
GlaxoSmithKline
Several key players have pursued strategic collaborations and pipeline expansions to strengthen their market positions. In 2025, Merck partnered with biotech firms to accelerate the development of PD-1 activators, expanding their product portfolio and increasing market share by 12%. Similarly, Bristol-Myers Squibb focused on acquisitions of novel immune checkpoint modulators, enhancing their competitive edge and driving revenue growth of nearly 15% in 2026. These strategic initiatives, including partnerships, mergers, and targeted R&D investments, have allowed leading companies to diversify offerings, stay ahead in innovation, and capture larger shares of the growing immune check activators market.
Immune Check Activators Market Future Outlook
The Immune Check Activators market is poised for robust growth in the coming years, driven by expanding oncology applications, rising autoimmune disease research, and the development of multi-targeted therapies. Advances in precision medicine, biomarker-driven patient stratification, and AI-enabled drug discovery will accelerate personalized treatment approaches. Emerging targets such as LAG-3, TIGIT, and novel checkpoint pathways are expected to diversify the therapeutic pipeline. Geographic expansion, particularly in Asia Pacific, along with supportive regulatory frameworks and increased healthcare spending, will further fuel adoption. Overall, continued innovation, strategic collaborations, and broader clinical applications are set to shape a dynamic and high-growth market landscape.
Immune Check Activators Market Historical Analysis
The Immune Check Activators market has experienced steady growth over the past decade, primarily driven by advances in cancer immunotherapy and the approval of PD-1 and CTLA-4 activators. Early clinical successes demonstrated the potential of immune checkpoint modulation, prompting increased R&D investments and the emergence of novel targets such as LAG-3 and TIGIT. Hospitals and specialty clinics became key end users as treatment adoption expanded. North America led the market historically due to strong healthcare infrastructure, regulatory support, and high patient awareness, while Europe followed closely with robust clinical research. These historical trends laid the foundation for the market’s rapid expansion in recent years.
Sources
Primary Research Interviews:
Oncologists
Immunologists
Clinical Trial Investigators
Biotech and Pharmaceutical R&D Professionals
Hospital Pharmacy and Specialty Clinic Managers
Databases:
FDA and EMA Databases
WHO Health Statistics
IQVIA
Magazines:
Cancer Therapy Advisor
The Scientist (Biotech Section)
BioCentury
Pharmaceutical Executive
Immuno-Oncology News
Journals:
Journal of Immunotherapy
Cancer Immunology Research
Frontiers in Immunology
Clinical Cancer Research
Nature Reviews Drug Discovery
Newspapers:
The New York Times (Health)
The Guardian (Health)
Financial Times (Healthcare)
The Hindu (Health)
Reuters Health
Associations:
American Society of Clinical Oncology (ASCO)
International Immuno-Oncology Society (ITMO)
Cancer Research Institute
European Society for Medical Oncology (ESMO)
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients