Hyperphosphatemia Drugs Market Size and Forecast – 2025 – 2032
The Global Hyperphosphatemia Drugs Market size is estimated to be valued at USD 3.6 billion in 2025 and is expected to reach USD 6.7 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 9.2% from 2025 to 2032.
Global Hyperphosphatemia Drugs Market Overview
Hyperphosphatemia drugs are therapeutic agents used to reduce elevated phosphate levels, primarily in patients with chronic kidney disease (CKD) undergoing dialysis. The market consists of phosphate binders such as calcium-based, non-calcium-based (sevelamer, lanthanum carbonate), and iron-based formulations. These products act by binding dietary phosphate in the gastrointestinal tract, preventing its absorption into the bloodstream. Newer therapies are focused on improving phosphate-binding efficiency and reducing pill burden.
Additionally, combination therapies and novel non-binder drugs targeting phosphate transport pathways are under development. The market continues to evolve with the increasing CKD prevalence and the shift toward non-calcium binders to mitigate cardiovascular risks, supported by a strong pipeline of next-generation phosphate control agents.
Key Takeaways
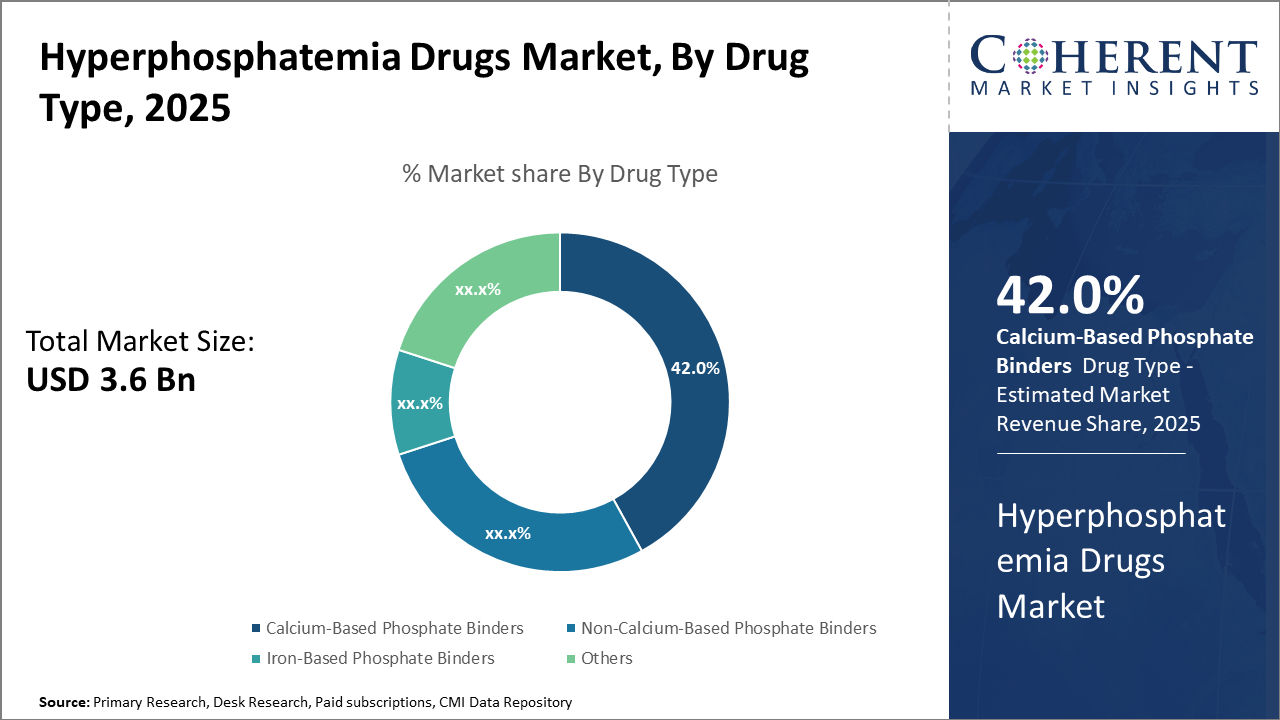
The calcium-based phosphate binder segment dominates the market share, accounting for 42%, primarily driven by its cost-effectiveness and extensive clinical use in dialysis patients.
Meanwhile, iron-based phosphate binders emerge as the fastest-growing subsegment due to their combined therapeutic benefits and increasing adoption in non-dialysis CKD patients.
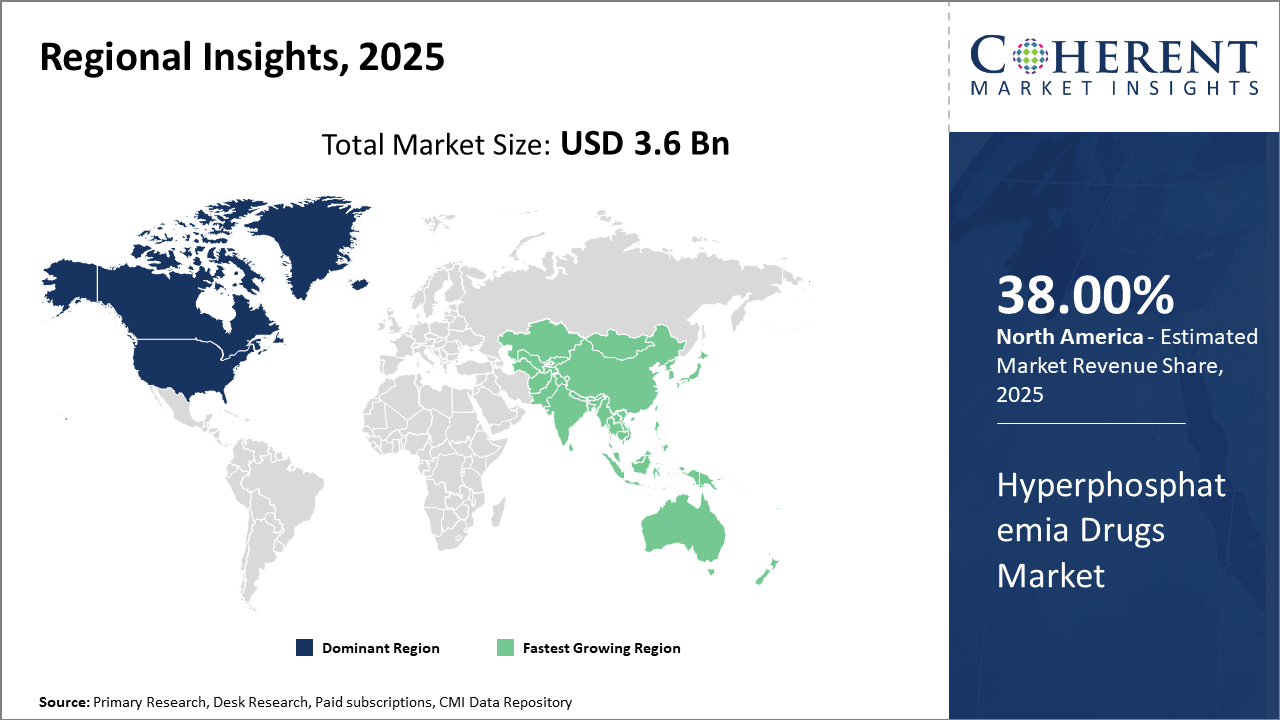
Regionally, North America leads the market with approximately 38% market share, attributable to well-established healthcare infrastructure and high patient awareness.
The Asia Pacific region is the fastest-growing market, recording a CAGR exceeding 10%, fueled by improving healthcare expenditures and demographic trends such as rising CKD prevalence in India and China.
Hyperphosphatemia Drugs Market Segmentation Analysis
To learn more about this report, Download Free Sample
Hyperphosphatemia Drugs Market Insights, By Drug Type
Calcium-Based Phosphate Binders dominate the market share with 42%. Calcium-based binders remain dominant due to their cost-effectiveness and long-standing clinical acceptance, which make them a preferred choice in dialysis settings. However, the fastest-growing subsegment is Iron-Based Phosphate Binders, which are gaining momentum because of their dual action in managing anemia and serum phosphate levels, addressing multiple complications in CKD patients, and improving patient adherence.
Hyperphosphatemia Drugs Market Insights, By Application
Dialysis Patients constitute the dominating subsegment because their elevated phosphate levels necessitate ongoing medical management, rendering this group the primary revenue source for market players. Conversely, Non-Dialysis CKD Patients represent the fastest-growing subsegment as early intervention trends gain traction, with evolving treatment guidelines recommending phosphate management before dialysis initiation. Pediatric Patients represent a niche market segment focused on specific formulations catering to this vulnerable group, while Others include off-label or emerging use cases with currently limited penetration.
Hyperphosphatemia Drugs Market Insights, By Distribution Channel
Hospital Pharmacies dominate the market share thanks to direct drug dispensing in dialysis centers and specialized nephrology clinics, ensuring controlled usage and compliance for complex therapies. Online Pharmacies are the fastest-growing channel segment, propelled by digital health penetration and increasing patient preference for convenience, particularly post-pandemic, driving wider availability and timely delivery. Retail Pharmacies maintain steady demand by servicing routine prescriptions, while Others encompass emergent channels such as direct-to-patient shipping services and clinical trial distributions that are in nascent stages.
Hyperphosphatemia Drugs Market Trends
Market trends in hyperphosphatemia drugs emphasize a movement towards incorporating multifunctional therapies combining phosphate control with anemia management, driven by clinical evidence emerging in 2024 and 2025.
The adoption of iron-based phosphate binders reflects this, with new product launches in North America showcasing multifunctional efficacy and gaining significant market traction.
Likewise, digital integration through apps and remote monitoring in managing phosphate levels enhances clinical outcomes and treatment adherence, demonstrating a shift towards tech-enabled healthcare in nephrology.
Hyperphosphatemia Drugs Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Hyperphosphatemia Drugs Market Analysis and Trends
In North America, the hyperphosphatemia drugs market commands approximately 38% of the total industry share. This dominance is supported by a mature healthcare ecosystem, extensive dialysis patient base, and proactive reimbursement policies. Notable companies like Amgen and Vifor Pharma leverage strong distribution networks and continuous clinical innovation to maintain leadership. The presence of advanced regulatory frameworks facilitates the swift market introduction of new formulations, attracting significant investments.
Asia Pacific Hyperphosphatemia Drugs Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with a CAGR exceeding 10%. Rising CKD prevalence in China and India, alongside expanding healthcare infrastructure, is pivotal. Government initiatives improving dialysis access and reimbursement, coupled with increasing awareness of mineral metabolism complications, are propelling market expansion. Domestic market players and multinationals have been actively collaborating to increase drug availability and affordability, thereby enhancing business growth in the region.
Hyperphosphatemia Drugs Market Outlook for Key Countries
USA Hyperphosphatemia Drugs Market Analysis and Trends
The USA accounts for a substantial share of the hyperphosphatemia drugs market, driven by the high incidence of end-stage renal disease (ESRD). According to data from the USRDS, over 785,000 Americans were on dialysis in 2024, directly fueling pharmaceutical demand for phosphate management. With robust healthcare expenditure exceeding $4 trillion annually, the nation supports the continuous adoption of novel therapies. Leading companies such as Amgen and Akebia Therapeutics have accelerated clinical studies and launched new phosphate binder formulations, contributing to increased market revenue and refined therapeutic protocols.
India Hyperphosphatemia Drugs Market Analysis and Trends
India’s hyperphosphatemia drugs market is rapidly expanding due to an escalating CKD patient population, estimated at over 100 million affected individuals in recent epidemiological studies. The government healthcare initiatives aimed at improving renal care infrastructure and subsidizing essential drug therapies have positioned the market for double-digit growth. Domestic players, alongside multinational corporations, actively participate in expanding affordable phosphate binder availability. Surge in urbanization and healthcare awareness contributes to increased diagnosis and treatment adherence, making India a critical market for future business growth.
Analyst Opinion
The rise in dialysis patients worldwide acts as a strong demand-side indicator expanding the hyperphosphatemia drugs market share. According to the United States Renal Data System (USRDS), nearly 785,000 individuals in the U.S. required dialysis or kidney transplantation in 2024, marking a 3.1% increase from the previous year, thereby driving demand for phosphate binders to control serum phosphate levels.
Innovation in drug formulations, including oral non-calcium phosphate binders and iron-based phosphate binders, has enhanced patient compliance and safety profiles. In 2025, sales of sevelamer and lanthanum carbonate formulations increased by 12% and 15%, respectively, indicating strong market revenue contributions from improved therapeutic options.
Pricing strategies emphasizing value-based purchasing and reimbursement acceleration in emerging markets bolster accessibility and adoption rates. For example, policy changes in India expanded coverage for phosphate binder therapies in public health schemes in late 2024, contributing to a projected 14% year-on-year market growth in Asia Pacific.
The import-export dynamics reflect a growing international trade of hyperphosphatemia drugs, with exports from North America to Asia Pacific increasing by 9% in 2024 due to heightened demand in fast-developing healthcare economies. This trend directly impacts regional market dynamics and forecasts.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: |
USD 3.6 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.2% | 2032 Value Projection: | USD 6.7 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Amgen Inc., Shire Pharmaceuticals, Vifor Pharma, Akebia Therapeutics, Kyowa Kirin Co. Ltd., Nipro Corporation, Baxter International Inc., Taiho Pharmaceutical Co. Ltd., Takeda Pharmaceutical Company Limited, Otsuka Holdings Co., Ltd. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Hyperphosphatemia Drugs Market Growth Factors
Rapid growth in the global dialysis population remains a critical growth driver, directly correlating with hyperphosphatemia drug demand. Increased diagnosis and treatment rates in advanced CKD stages propel market revenue further. Secondly, improved reimbursement frameworks across countries are enhancing access to advanced phosphate binders, stimulating market share expansion in emerging regions. Thirdly, evolving clinical guidelines emphasizing early intervention for phosphate management support sustained market growth strategies. Lastly, the rise in healthcare expenditure and infrastructure development in Asia Pacific and Latin America are fueling the overall market forecast, with emerging players expanding their presence.
Hyperphosphatemia Drugs Market Development
In October 2023, XPHOZAH® (tenapanor) received U.S. FDA approval, marking the first-in-class phosphate absorption inhibitor indicated to reduce serum phosphorus in adults with chronic kidney disease (CKD) on dialysis who have had an inadequate response to phosphate binders or are intolerant to any dose of phosphate binders. The approval addresses a significant unmet need in renal mineral-bone disease management.
In June 2025, Alebund Pharmaceuticals announced positive topline results from its Phase III trial of AP301, an investigational oral fiber-iron-based phosphate binder designed for dialysis patients with hyperphosphatemia. The study met its primary endpoint, showing statistically significant and clinically meaningful improvement in serum phosphorus control. The outcome positions AP301 as a potential future launch candidate in the dialysis-phosphate-management market.
Key Players
Leading Companies of the Market
Amgen Inc.
Shire Pharmaceuticals
Vifor Pharma
Akebia Therapeutics
Kyowa Kirin Co. Ltd.
Nipro Corporation
Baxter International Inc.
Taiho Pharmaceutical Co. Ltd.
Takeda Pharmaceutical Company Limited
Otsuka Holdings Co., Ltd.
Several leading companies have adopted growth strategies such as strategic acquisitions and collaborations to strengthen their product portfolios. For instance, Vifor Pharma’s acquisition by a global pharmaceutical giant in 2024 significantly expanded its research capabilities and distribution network within the hyperphosphatemia drugs market. Similarly, Akebia Therapeutics’ late-stage clinical trial collaboration in 2023 improved its innovative drug pipeline, positioning the company favorably in capturing emerging market opportunities.
Hyperphosphatemia Drugs Market Future Outlook
The future of the hyperphosphatemia drugs market is shaped by continuous innovation in drug formulations, reduced pill burden, and patient-centric therapy design. Research is moving toward novel non-binder drugs that inhibit intestinal phosphate transporters, providing a more physiological approach to phosphate management. Fixed-dose combinations and chewable formulations are expected to enhance compliance among dialysis patients. As healthcare systems focus on integrated CKD management, the market will see more partnerships between pharmaceutical and dialysis service providers. Moreover, emerging economies are expected to drive volume growth due to the increasing availability of affordable generics. The focus on precision dosing, improved tolerability, and minimal gastrointestinal side effects will define the next generation of hyperphosphatemia therapeutics.
Hyperphosphatemia Drugs Market Evolaution
Historically, the hyperphosphatemia drugs market has evolved alongside advances in nephrology and dialysis care. In the early 1990s, calcium-based phosphate binders dominated treatment regimens, offering cost-effective phosphate control but raising long-term cardiovascular concerns. The early 2000s saw the introduction of non-calcium-based binders such as sevelamer and lanthanum carbonate, which improved safety profiles and patient adherence. Over time, iron-based binders such as ferric citrate and sucroferric oxyhydroxide expanded therapeutic choices, offering dual benefits of phosphate control and anemia management. With chronic kidney disease (CKD) incidence rising globally, especially among diabetic and hypertensive populations, hyperphosphatemia management became a standard component of dialysis therapy. Stringent treatment guidelines by organizations such as KDIGO further strengthened the use of phosphate binders as essential supportive drugs.
Sources
Primary Research Interviews:
Nephrologists
Dialysis Center Directors
Pharmaceutical R&D Scientists
Hospital Pharmacists
Databases:
ClinicalTrials.gov
WHO CKD Data
FDA Drug Database
Magazines:
Nephrology News & Issues
Drug Development & Delivery
Pharma Times
Kidney International Insights
Journals:
American Journal of Kidney Diseases
Nephrology Dialysis Transplantation
Clinical Nephrology
Kidney Medicine
Associations:
National Kidney Foundation (NKF)
American Society of Nephrology (ASN)
International Society of Nephrology (ISN)
European Renal Association (ERA)
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients