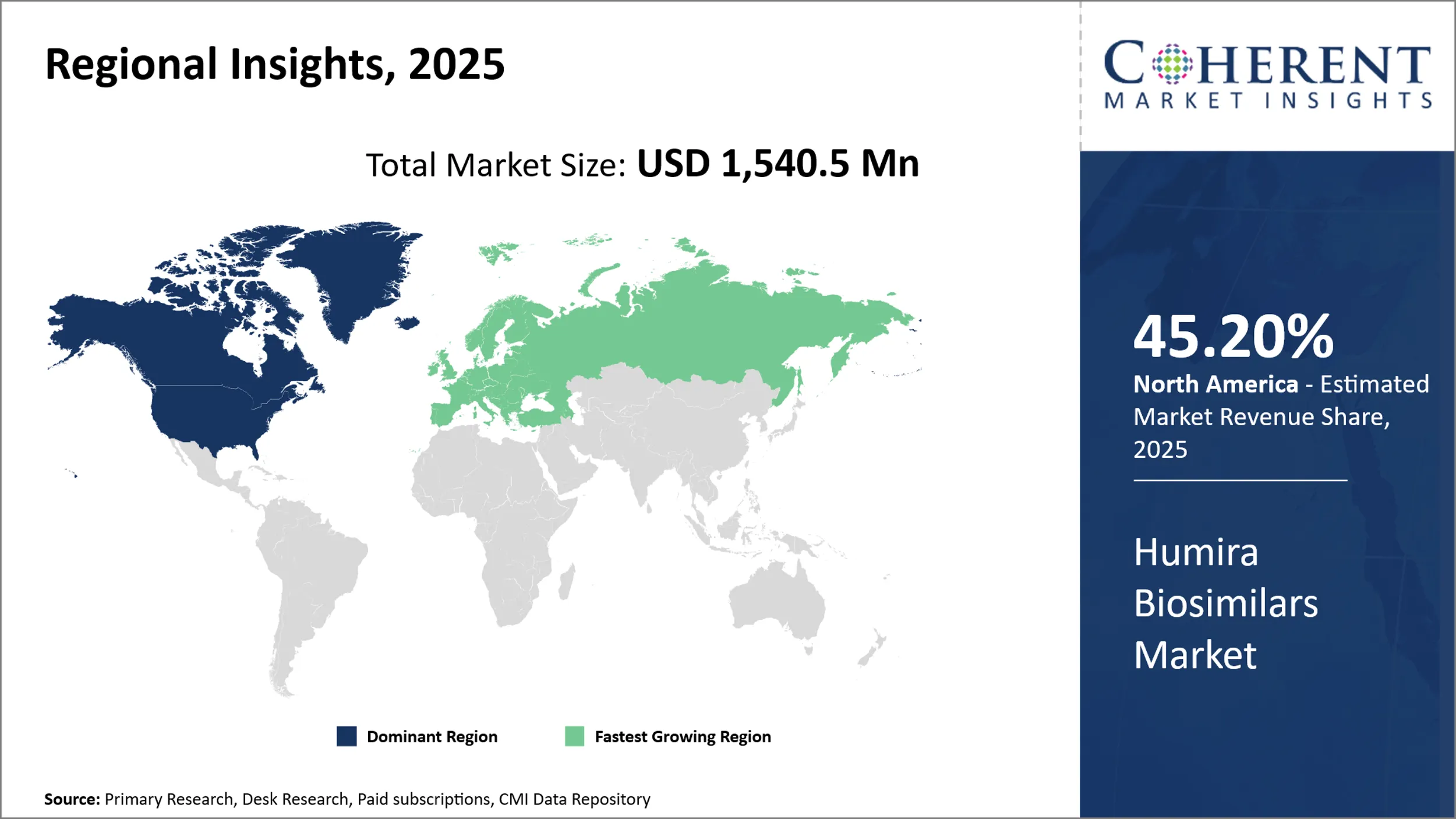
Humira Biosimilar Market is estimated to be valued at USD 1,540.5 Mn in 2025 and is expected to reach USD 7,724.0 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 25.9% from 2025 to 2032.
The surge in demand for low-cost substitutes to the original Humira medication on the market for treating autoimmune diseases has spike the fill of Amgen which launched its Amjevita biosimilar worldwide in 2024. Increased biosimilar awareness and the expiration of numerous patents are propelling market growth. However, regulatory obstacles and physician acceptance may slow down the rate of adoption.
Humira is a biologic drug used to treat various autoimmune diseases, including rheumatoid arthritis and psoriasis, holds the distinction of being the top-selling biologic drug globally, generating annual sales exceeding USD 20 billion.
|
Event |
Description and Impact |
|
U.S. Patent Cliff and Market Entry Timeline |
|
|
European Biosimilar Market Maturation and Pricing Dynamics |
|
|
China's Biosimilar Regulatory Reforms and Market Access |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
By product type, the Biosimilar Humira segment is expected to lead the global Humira biosimilar market with a commanding share of 33.5% in 2025. This leading position is driven by increasing adoption of cost-effective alternatives to the original Humira biologic, especially in regions with strong regulatory support for biosimilars.
The biosimilar segment benefits from growing awareness among healthcare providers and patients about biosimilars’ comparable efficacy and safety profiles, making it a preferred choice for chronic conditions such as rheumatoid arthritis and psoriasis.
The Original Humira segment, while still significant, faces challenges from patent expirations and competitive pricing pressures, which accelerate the uptake of biosimilars. However, its established brand presence and extensive clinical data continue to support steady demand in some markets.
Emerging New Formulations or Next-Generation Products are also contributing to the market, driven by innovations aiming to improve patient convenience through alternative delivery methods or enhanced efficacy. These new products, although currently holding a smaller share, are expected to gain traction over the forecast period.
The Others category includes various ancillary products and off-label biosimilar uses, catering to niche therapeutic applications or regional preferences.
To learn more about this report, Download Free Sample
North America, led predominantly by the U.S., holds a commanding 45.20% share of the global Humira biosimilar market in 2025. Although the introduction of biosimilars in this region faced initial challenges due to patent protections and complex regulatory frameworks, the expiration of Humira’s patents has unlocked significant growth opportunities.
The region’s well-established pharmaceutical industry, strong healthcare infrastructure, and rising demand for cost-effective autoimmune treatments underpin the increasing adoption of biosimilars. Ongoing efforts by regulatory agencies to streamline biosimilar approvals are further accelerating market expansion.
Europe stands as a pioneer in biosimilar adoption, capturing approximately 30.7% of the global market share in 2025. The region’s early establishment of clear regulatory pathways and supportive policies has fostered a competitive market landscape with multiple approved and launched Humira biosimilars.
European countries benefit from heightened biosimilar uptake driven by healthcare cost containment initiatives and growing physician acceptance. Germany, the U.K., and France lead the regional market, reflecting strong pharmaceutical innovation and robust healthcare systems.
Asia Pacific is poised for significant growth in the Humira biosimilar market, accounting for around 16.8% share in 2025. Countries such as Japan, South Korea, and Australia are witnessing increasing biosimilar penetration, propelled by the rising prevalence of autoimmune diseases and the pressing need for affordable treatment options.
While regulatory environments and market access vary across the region, ongoing improvements in approval processes and expanding healthcare infrastructure support growth. Emerging markets within Asia Pacific also present untapped potential for biosimilar adoption.
The U.S. is the largest market in North America, driven by high healthcare expenditure, increasing patient awareness, and expanding biosimilar acceptance following patent expirations. The U.S. regulatory framework is evolving to facilitate biosimilar market entry.
Canada’s growing biosimilar market benefits from government initiatives promoting cost-effective healthcare and increasing access to biosimilar therapies.
Germany leads Europe’s Humira biosimilar market, supported by early biosimilar adoption, favorable reimbursement policies, and a strong pharmaceutical industry.
The U.K. exhibits rapid biosimilar uptake driven by NHS cost-saving programs and progressive regulatory guidance.
France remains a key European market with expanding biosimilar penetration fueled by healthcare reforms and increasing patient access.
Japan’s expanding pharmaceutical market and rising autoimmune disease prevalence underpin growth in the Humira biosimilar segment, despite cautious regulatory pathways.
South Korea’s biosimilar market is growing due to government support, increasing healthcare expenditure, and improving regulatory frameworks.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1,540.5 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 25.9% | 2032 Value Projection: | USD 7,724.0 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Amgen Inc., Samsung Bioepis Co., Ltd., Sandoz International GmbH (Novartis AG), Mylan N.V., Boehringer Ingelheim International GmbH, Pfizer Inc., Fresenius Kabi AG, Coherus BioSciences, Inc., Biogen Inc., AbbVie Inc., Celltrion Inc., Rani Therapeutics Holdings, Inc., Teva Pharmaceutical Industries Ltd, Merck & Co., Inc., Viatris Inc., and Alvotech |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The expiration of the patents protecting humira paved the way for the entry of biosimilars. Once the patents expire, it allows other pharmaceutical companies to develop and market biosimilar versions of humira, creating competition in the market.
Biosimilars offer a more cost-effective alternative to the reference biologic drug. With the increasing demand for affordable healthcare, biosimilars provide an opportunity to reduce treatment costs for patients, healthcare systems, and payers. This cost-saving potential is a significant driver for the adoption of humira biosimilars. For instance, in October 2023, Celltrion USA, Inc., a biopharmaceutical company, announced its FDA-approved biosimilar, YUFLYMA (adalimumab-aaty), has been added to CarePartners Specialty Pharmacy Cost Savings Programs.
YUFLYMA is a high-concentration (100mg/mL) and citrate-free formulation of the Humira (adalimumab) biosimilar. CarePartners and its strategic partners will offer and distribute YUFLYMA as the lowest net cost high-concentration Humira (adalimumab) biosimilar to over 10 million plan members.
Autoimmune diseases, such as rheumatoid arthritis, psoriasis, and crohn's disease, are on the rise globally. The growing prevalence of these conditions drives the demand for effective and accessible treatments, including biosimilars of humira, which are proven therapies for autoimmune diseases.
Regulatory agencies in various regions had established guidelines and pathways for the approval and market access of biosimilars. These frameworks provide clarity and facilitate the development, evaluation, and commercialization of humira biosimilars. Favorable regulatory environments encourage pharmaceutical companies to invest in the development of biosimilars.
The availability of humira biosimilars offers an opportunity to improve access to treatment for patients with autoimmune diseases. Biosimilars provide a more affordable alternative to the reference biologic drug, allowing a broader population to benefit from effective therapies.
The introduction of humira biosimilars creates competition in the market, thereby driving innovation and cost optimization. This competition encourages pharmaceutical companies to develop and launch biosimilars, thus leading to a more competitive landscape. It also promotes market growth and the development of a diverse range of treatment options for patients.
The availability of humira biosimilars expands the range of treatment choices for physicians and patients. Physicians can select from multiple biosimilar options based on factors such as patient needs, pricing, and clinical evidence. Patients can benefit from a wider selection of therapies which are tailored for their specific conditions and preferences.
The humira biosimilar market presents opportunities for global expansion. As regulatory pathways and guidelines for biosimilars continue to evolve and improve worldwide, pharmaceutical companies can seek approvals in various regions, thus tapping into new markets and reaching a larger patient population.
Regulatory agencies worldwide have established pathways and guidelines for the approval and market entry of biosimilars, including those for humira. The number of regulatory approvals for humira biosimilars has been growing, allowing for expanded market availability and adoption.
The humira biosimilar market is expanding globally, with biosimilars becoming available in various regions. Europe has been at the forefront of biosimilar adoption, with a relatively higher number of approved humira biosimilars. However, other regions, including North America, Asia Pacific, and Latin America, are also witnessing the entry and growth of humira biosimilars.
As physicians gain more experience and confidence in prescribing biosimilars, biosimilar acceptance and adoption are increasing. Patient acceptance of biosimilars is also growing, driven by factors such as positive clinical outcomes, cost savings, and improved access to treatment options.
Collaborative partnerships between pharmaceutical companies, healthcare providers, and payers are emerging to drive the development, market access, and adoption of humira biosimilars. Such collaborations aim to improve patient access, education, and awareness about biosimilars, as well as address potential barriers to their uptake.
*Definition: Humira biosimilars are highly similar versions of the biologic drug humira (adalimumab) that have been developed and approved following the expiration of humira's patents. These biosimilars are designed to have comparable efficacy, safety, and quality to humira and provide more affordable treatment options for patients with autoimmune diseases such as rheumatoid arthritis, psoriasis, and crohn's disease.
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients