Human Papillomavirus Infection Vaccines Market Size and Forecast – 2025 – 2032
The Global Human Papillomavirus Infection Vaccines Market size is estimated to be valued at USD 6.8 billion in 2025 and is expected to reach USD 13.4 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 10.2% from 2025 to 2032.
Global Human Papillomavirus Infection Vaccines Market Overview
HPV vaccines are immunological products designed to prevent infection from high-risk HPV strains that can lead to cervical, anal, and other cancers. These vaccines are generally recombinant, virus-like particle (VLP)-based formulations that stimulate the immune system without containing live virus. Administered via intramuscular injection in multi-dose schedules, vaccines target specific HPV types (such as 6, 11, 16, and 18) and are available as bivalent, quadrivalent, and nonavalent options. Advanced formulations focus on broader protection, long-term immunity, and reduced dosing regimens for improved patient compliance.
Key Takeaways
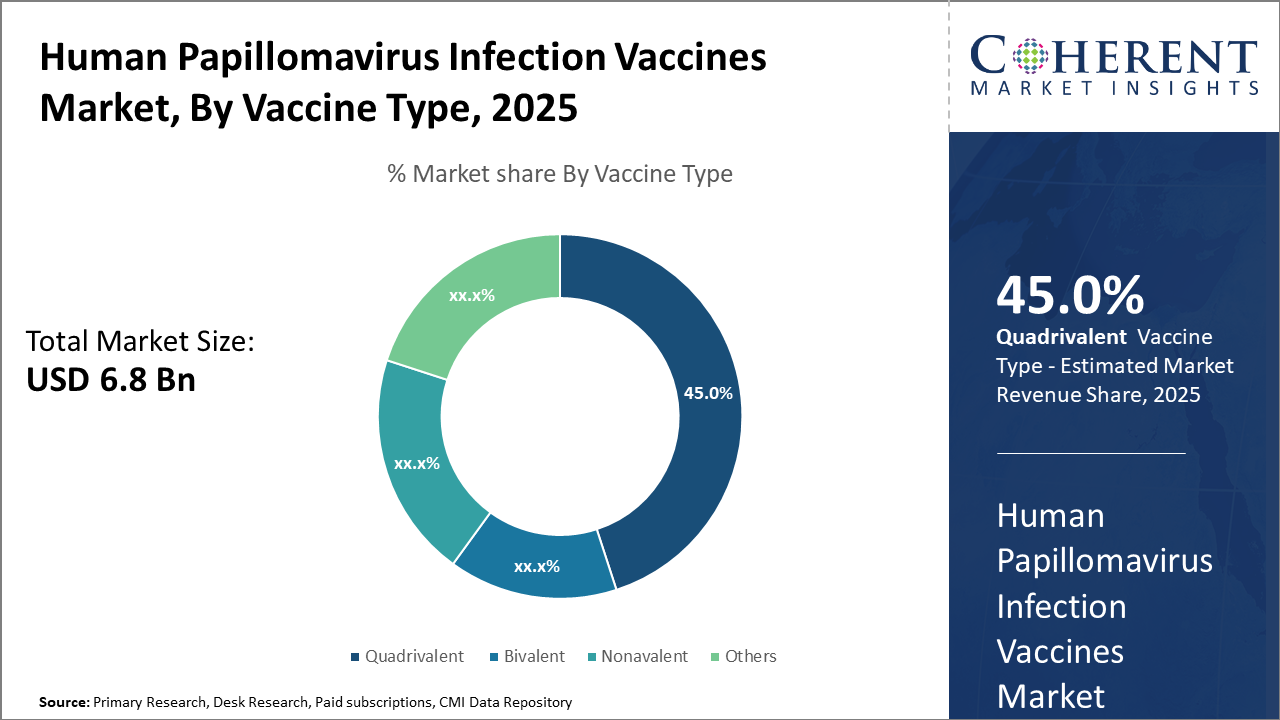
Quadrivalent vaccines maintain market leadership with a 45% share, driven by broad protection against multiple HPV strains and strong adoption in developed economies.
Nonavalent vaccines are the fastest-growing segment, attributed to expanding approvals and enhanced strain coverage.
The Hospitals & Clinics segment dominates end-user contributions with a 60% share, supported by established immunization infrastructure and high patient throughput. Government immunization programs exhibit rapid expansion owing to large-scale public health initiatives.
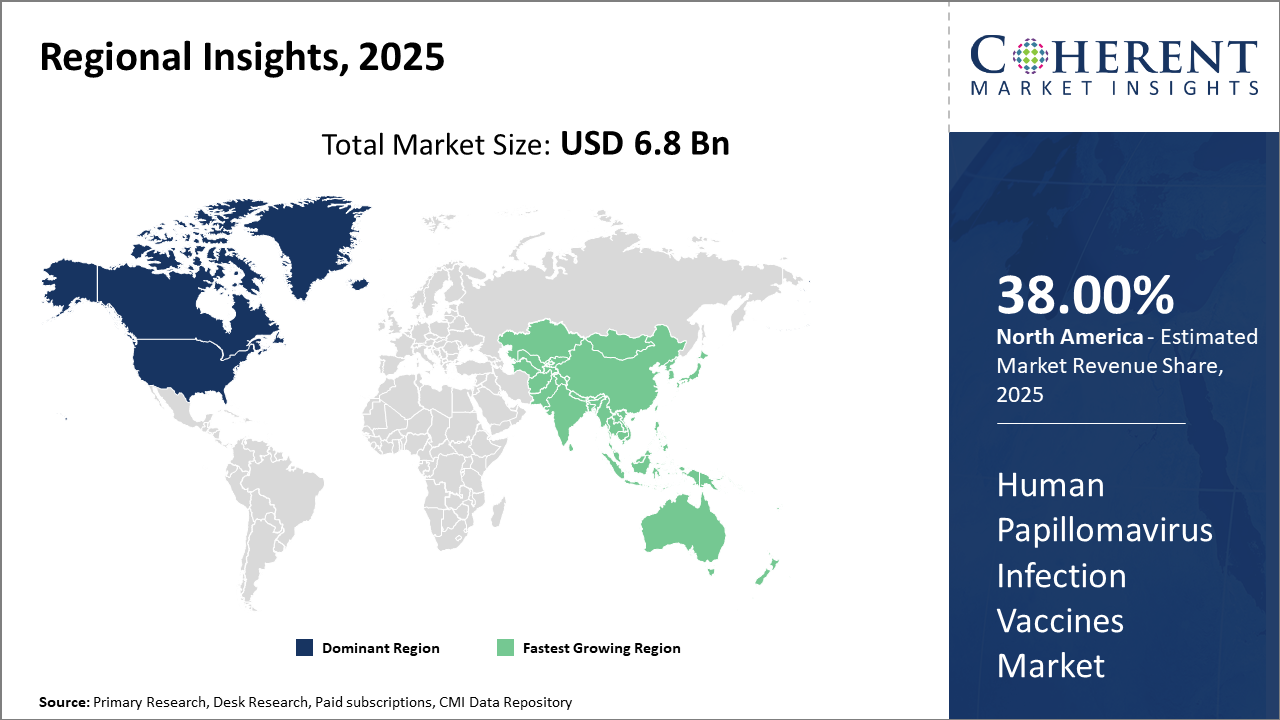
North America commands dominance in the Human Papillomavirus Infection Vaccines market at 38%, supported by advanced healthcare infrastructure and proactive vaccination policies.
The Asia Pacific region, driven by a growing population and improving healthcare access, is the fastest-growing market, with an impressive CAGR reflecting increased governmental focus on HPV awareness.
Human Papillomavirus Infection Vaccines Market Segmentation Analysis
To learn more about this report, Download Free Sample
Human Papillomavirus Infection Vaccines Market Insights, By Vaccine Type
In terms of vaccine type, Quadrivalent dominates the market share with 45%, due to its established clinical efficacy against four HPV strains responsible for the highest infection rates globally, facilitating widespread adoption, particularly in developed countries. The Nonavalent segment is the fastest growing, owing to its broader strain coverage and recent regulatory approvals in several countries, catering to growing prevention needs and supporting market expansion. Bivalent vaccines offer targeted protection against two high-risk HPV types, maintaining a steady demand in niche markets.
Human Papillomavirus Infection Vaccines Market Insights, By End-User
Hospitals & Clinics dominate with a 60% market share as primary vaccination points supported by robust healthcare infrastructure and comprehensive patient management systems. Government Immunization Programs represent a rapidly growing segment driven by public health policies aiming for blanket immunization coverage, especially in emerging economies. Private Clinics cater to specialized and affluent clientele, contributing to a steady but moderate market share.
Human Papillomavirus Infection Vaccines Market Insights, By Distribution Channel
Direct Sales prevail as the leading distribution route, driven by exclusive contracts between vaccine manufacturers and healthcare institutions, ensuring steady supply chains. Retail Pharmacies contribute to growing market penetration, especially in urban regions where consumer convenience is paramount. Online Channels are emerging rapidly, propelled by digital health trends and increasing consumer comfort with e-pharmacy services, representing the fastest-growing subsegment.
Human Papillomavirus Infection Vaccines Market Trends
The Human Papillomavirus Infection Vaccines market continues to evolve with a pronounced shift towards inclusivity beyond female-centric programs.
For example, recent epidemiological data from 2025 show growing prevalence of HPV-related oropharyngeal cancers in males, prompting expanded vaccine recommendations.
Simultaneously, single-dose vaccine efficacy studies are transforming routine vaccination logistics by potentially reducing dosage requirements without compromising immunity.
This switch could significantly reduce program costs and improve compliance rates, especially in resource-limited settings.
Another emerging trend is the integration of digital health platforms to promote vaccination scheduling and awareness campaigns, resulting in increased coverage and more precise market segmentation.
Human Papillomavirus Infection Vaccines Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Human Papillomavirus Infection Vaccines Market Analysis and Trends
In North America, the dominance in the Human Papillomavirus Infection Vaccines market is driven by the U.S., which contributes the largest industry share globally. Well-established immunization infrastructure combined with government policies targeting near-universal adolescent coverage have propelled the market. Leading companies conduct extensive clinical trials in this region, ensuring rapid adoption of innovations. Proactive insurance reimbursement policies and strong public health advocacy have maintained high household and institutional vaccination rates.
Asia Pacific Human Papillomavirus Infection Vaccines Market Analysis and Trends
The Asia Pacific exhibits the fastest growth, fueled by expanding government immunization programs and improving healthcare delivery. Countries such as India and China are witnessing considerable investment in vaccine production and distribution, while population scale presents vast untapped opportunities. Strategic collaborations between local manufacturers and multinational market players help scale vaccination access, underpinning the region’s high CAGR. Government subsidies and growing public awareness campaigns further amplify growth prospects.
Human Papillomavirus Infection Vaccines Market Outlook for Key Countries
USA Human Papillomavirus Infection Vaccines Market Analysis and Trends
The U.S. market remains pivotal, driven by extensive vaccination recommendations that have resulted in over 75% coverage among girls and boys aged 13 to 17, as reported by the CDC in 2024. Merck's Gardasil and GlaxoSmithKline’s Cervarix dominate market revenue, supported by strong clinical evidence favoring quadrivalent and nonavalent vaccines. The U.S. government’s Vaccines for Children (VFC) program plays a critical role in maintaining demand by subsidizing immunizations, further stabilizing market dynamics despite occasional vaccine hesitancy challenges.
India Human Papilloma virus Infection Vaccines Market Analysis and Trends
India’s market is experiencing rapid growth, propelled by rising government focus on HPV awareness and vaccine accessibility. The Serum Institute of India and Bharat Biotech actively contribute with cost-effective vaccines, benefiting from local manufacturing efficiencies. Nationwide initiatives to include HPV vaccines within routine immunization schedules have accelerated uptake, especially in urban centers. Collaboration with global health organizations has improved funding and supply chains, positioning India as a major emerging market with significant potential.
Analyst Opinion
Demand-side dynamics remain a crucial driver with rising vaccination uptake among adolescents and young adults. For instance, the Centers for Disease Control and Prevention (CDC) reported a 16% increase in HPV vaccine coverage among U.S. teens in 2024, emphasizing growing public demand as a market catalyst.
Pricing strategies across leading market players demonstrate an increasing focus on competitive cost structures to boost accessibility. Recent price reductions in vaccination programs in low and middle-income countries under the GAVI alliance highlight tangible effects on market revenue expansion.
Production capacity enhancements within vaccine manufacturing hubs in Asia, particularly India and China, contributed to a 12% surge in global supply volume during 2024, indicating robust supply-side support facilitating market growth.
Expanding applications of HPV vaccines beyond cervical cancer prevention to other HPV-related malignancies, such as oropharyngeal cancer, have diversified market use cases. Clinical trial results published in 2025 substantiate improved vaccine efficacy in males, broadening the target demographic and boosting industry trends.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 6.8 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.2% | 2032 Value Projection: | USD 13.4 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Merck & Co., Inc., GlaxoSmithKline plc, Serum Institute of India Pvt. Ltd., Walvax Biotechnology Co., Ltd., Sanofi Pasteur, Bharat Biotech International Ltd., Pfizer Inc., Zhejiang Hisun Pharmaceutical Co., Ltd., Johnson & Johnson, Bio Farma, Panacea Biotec. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Human Papillomavirus Infection Vaccines Market Growth Factors
Increasing government initiatives for national immunization programs have substantially elevated HPV vaccination rates, with countries like Australia achieving over 80% vaccine coverage in 2024, fueling market dynamics. Rising awareness campaigns focusing on HPV infection risks and the benefits of prophylactic vaccines have amplified demand, especially in urban areas across North America and Europe. Additionally, evolving vaccine technology with longer-lasting immunogenicity profiles has enhanced market growth potentials as stakeholders prefer these advanced formulations. Moreover, affordability improvements through public-private partnerships and subsidy schemes in emerging countries have addressed significant market restraints, paving the way for sustained business growth.
Human Papillomavirus Infection Vaccines Market Development
In January 2023, the Serum Institute of India (SII), in collaboration with the Department of Biotechnology (DBT), launched CERVAVAC, India’s first indigenously developed quadrivalent human papillomavirus (HPV) vaccine. Designed to protect against HPV types 6, 11, 16, and 18, CERVAVAC provides comprehensive protection against both cervical cancer–causing and wart-causing HPV strains. The vaccine uses virus-like particles (VLPs) produced through recombinant DNA technology, ensuring high immunogenicity without containing any infectious material.
In October 2024, the World Health Organization (WHO) granted prequalification to Walrinvax®, the fifth HPV vaccine to achieve this global quality benchmark. Developed through collaborative efforts between Chinese biopharmaceutical firms and international research partners, Walrinvax® is designed to be cost-effective and suitable for use in large-scale immunization campaigns.
Key Players
Leading companies of the market include:
Merck & Co., Inc.
GlaxoSmithKline plc
Serum Institute of India Pvt. Ltd.
Sanofi Pasteur
Bharat Biotech International Ltd.
Zhejiang Hisun Pharmaceutical Co., Ltd.
Johnson & Johnson
Bio Farma
Panacea Biotec
Competitive strategies are heavily focused on broadening product portfolios through R&D investments, exemplified by Merck’s recent launch of enhanced nonavalent vaccines targeting additional HPV strains, which boosted their market share by 8% in 2024. Similarly, GlaxoSmithKline adopted strategic partnerships and licensing agreements in Southeast Asia, expanding immunization reach and witnessing a 15% revenue growth within that region during 2025's first half.
Human Papillomavirus Infection Vaccines Market Future Outlook
The future of HPV vaccination lies in enhancing global access, affordability, and immunization coverage, especially in developing nations where HPV-related cancer incidence remains high. Ongoing research aims to create broader-spectrum vaccines offering cross-protection and longer-lasting immunity, while simplified dosing schedules may improve compliance. Technological innovations such as thermostable formulations and needle-free delivery methods could expand vaccine distribution in low-resource settings. Collaborative global health initiatives, gender-neutral vaccination policies, and integration with public health programs are expected to make HPV vaccines a cornerstone of cancer prevention strategies worldwide.
Human Papillomavirus Infection Vaccines Market Historical Analysis
The development of HPV vaccines marked a major milestone in preventive medicine, emerging from decades of research linking specific HPV strains to cervical and other cancers. The first-generation vaccines were launched after rigorous clinical validation, providing targeted protection against the most oncogenic strains. Over time, improvements in vaccine formulations expanded coverage to additional HPV types, increasing effectiveness and long-term immunity. Global immunization campaigns, supported by healthcare agencies and public health organizations, facilitated large-scale vaccine adoption, significantly reducing infection rates and related diseases in several regions.
Sources
Primary Research interviews:
Vaccinologists
Public Health Officials
Immunologists
Vaccine Program Managers
Databases:
WHO Immunization Data
GAVI / Vaccine Alliance Resources
FDA / EMA Vaccine Approvals Databases
Magazines:
Vaccine News Daily
PharmaTimes
Vaccine Today
Global Health Now
Journals:
The Lancet Infectious Diseases
Journal of Virology
Human Vaccines & Immunotherapeutics
Newspapers:
The New York Times (Health)
The Guardian (Global Health)
The Washington Post (Health)
The Economic Times (Health)
Associations:
World Health Organization (WHO)
Gavi – The Vaccine Alliance
International Society for Vaccines (ISV)
National Immunization Technical Advisory Groups (NITAGs)
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients