Human Microbiome Market is estimated to be valued at USD 959.4 Mn in 2025 and is expected to reach USD 4,473.3 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 24.6% from 2025 to 2032.
Analysts’ Views on Global Human Microbiome Market:
Human microbiome includes all the bacteria, archaea, viruses, and eukaryotes that are present inside and outside bodies. The human microbiota consists of the 10-100 trillion symbiotic microbial cells in each individual. Increased awareness about prebiotics and probiotics and their role in preventive health measures are expected to drive the market growth. Rising geriatric population and growing prevalence of lifestyle diseases like cancer and autoimmune are expected to drive the market growth. The healthcare infrastructure of developing countries globally receives funding from government for bigger projects like ‘human microbiome project’ and ‘the earth Microbiome’. These bigger initiatives provides opportunities to market players for research and development new products in human microbiome that drives the market growth during the forecast period.
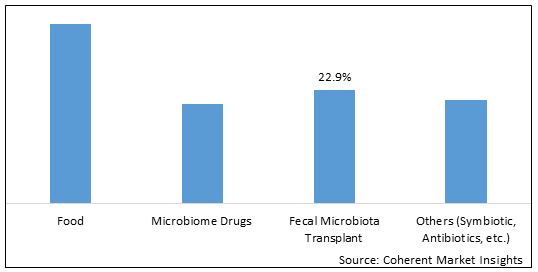
Figure 1. Global Human Microbiome Market Share (%), By Product Type, 2025
To learn more about this report, Download Free Sample
Global Human Microbiome Market– Drivers
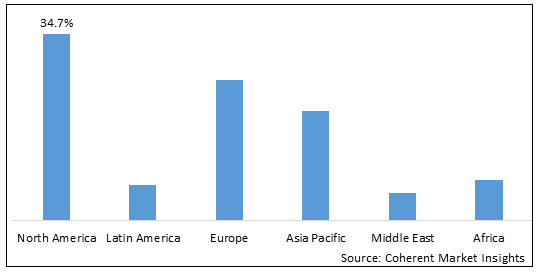
Figure 2. Global Human Microbiome Market Share(%), By Region, 2025
To learn more about this report, Download Free Sample
Global Human Microbiome Market- Regional Analysis
Among region, North America is estimated to hold a dominant position in the global human microbiome market over the forecast period. North America is estimated to hold 34.7 % of the market share in 2025. The global human microbiome market is expected to witness significant growth in the near future due to high prevalence of orthopedic disorders and dysfunctions, favorable health reimbursement, and increased awareness. Increasing prevalence of lifestyle diseases like cancer andautoimmune disorders contributes to growth of human microbiome market in North America region. For instance, in March 2022, according to an article published by Crohn’s & Colitis Foundation’s in Fact sheet magazine, around 1.6 and 3.1 million Americans suffer from IBD out of which 25% are below he age of 18 years as per the data published for year 2021.
Global Human Microbiome Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others faced problems regarding transportation of things from one place to another.
However, the COVID-19 pandemic had positive impact on the global human microbiome market, owing to increased researches on microbial association with human body. For instance, in March 2021, according to an article published by frontiers microbitechnology magazine, under the section infectious diseases, there are ongoing researches on direct relationships between the COVID-19 virus and the lung and gut microbiomes which can be bidirectional. Changing health and hygiene, changes to mood and diet can lead to enduring changes to the gut microbiome at an individual and a population level.
Human Microbiome Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 959.4 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 24.6% | 2032 Value Projection: | USD 4,473.3 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Seres Therapeutics, Biomesense, Microbiotica, Infant Bacterial Therapeutics AB, Vedanta Biosciences, Inc., Second genome therapeutics, 4D Pharma Ferring Inc, Enterome, BiomX, MaaT Pharma, Azitra, Illumina, Inc, Locus Biosciences, Inc, Finch Therapeutics Group, Inc, Rebiotix Inc, Servatus Ltd, Microbiome Research Pvt. Ltd, AOBiome, Axial Therapeutics, Inc, Biomica. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Human Microbiome Market- Segmentation
Global human microbiome market report is segmented into by product type, by disease indication, by application, by distribution channel, and by region.
Among all the segmentation, the microbiome drug segment is expected to dominate the market over the forecast period due to ongoing research and development in the microbiome based products and drugs and rising funding from bigger MNCs and Pharmaceutical companies to research institutes and smaller firms in this fields.
Global Human Microbiome Market- Cross Sectional Analysis
Key players are focusing on organic strategies such as the launch of new drug which is expected to drive expected to boost the microbiome drug segment in human microbiome market in North America region. For instance, on April 26, 2023, Seres Therapeutics, a commercial stage microbiome threpeutics manufacturing company, in collaboration with Nestlé Health Science, a global leading company in nutrition science, announced that the U.S. food and Drug Administration (FDA) had approved VOWST, first orally administered microbiota based therapeutic for the prevention of recurring C. difficile infection. These kinds of developments in the healthcare system and rising health expenditures are fueling the global human microbiome market growth. Increased number of imflamatory bowel disorder in the U.S and Canada is growing the human microbiome market in North America Region.
Global Human Microbiome Market: Key Developments
Global Human Microbiome Market: Key Trends
Global Human Microbiome Market: Restraint
Global Human Microbiome Market - Key Players
Major players operating in the global human microbiome market include Seres Therapeutics, Biomesense, Microbiotica, Infant Bacterial Therapeutics AB, Vedanta Biosciences, Inc., SECOND GENOME THERAPEUTICS, 4D Pharma Ferring Inc, Enterome, BiomX, MaaT Pharma, Azitra, Illumina, Inc, Locus Biosciences, Inc, Finch Therapeutics Group, Inc, Rebiotix Inc, Servatus Ltd, Microbiome Research Pvt. Ltd, AOBiome, Axial Therapeutics, Inc, Biomica.
*Definition: Human microbiome are the full range of microorganisms that are present inside and outside the Humans. The human micro biota consists of the 10-100 trillion symbiotic microbial cells primarily bacteria in the gut the human. These microbiomes are mostly are symbiotic that benefits human and some are pathogenic promoting disease. In a healthy body, pathogenic and symbiotic microbiota coexist without problems.
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients