Human Immunoglobulin (pH4) for Intravenous Injection Market is estimated to be valued at USD 78.37 Bn in 2025 and is expected to reach USD 182.1 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 12.8% from 2025 to 2032. For instance, according to the data provided by National Health Service- the publicly funded healthcare system in England, the immunoglobulin used to treat Kawasaki disease is called gamma globulin. After the administration of IVIG, symptoms of fever, flu, or rash improve within 36 hours.
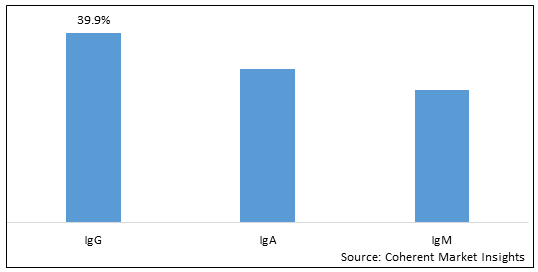
Figure 1. Global Human Immunoglobulin (pH4) for Intravenous Injection Market Share (%), by Disease Indication, 2025
To learn more about this report, Download Free Sample
Global Human Immunoglobulin (pH4) for Intravenous Injection Market – Drivers
Increasing incidence of disease outbreaks
The increasing incidence of disease outbreaks is escalating demand for advanced diagnostic measures, which is expected to boost the growth of the global human immunoglobulin (pH4) for intravenous injection market. For instance, the coronavirus disease (COVID-19) pandemic is the most recent outbreak which was first reported on January 1, 2020, in Wuhan, China. The World Health Organization declared coronavirus disease (COVID-19) as a pandemic on March 11, 2020. According to the World Health Organization (WHO), around 1,991,562 cases of the coronavirus disease (COVID-19) were reported on April 16, 2020, across the globe.
Increasing approval of intravenous human immunoglobulin products
Approvals of intravenous human immunoglobulin products by regulatory authorities are expected to drive the growth of the human immunoglobulin (pH4) for the intravenous injection market during the forecast period. For instance, on May 4, 2023, The U.S. Food and Drug Administration (FDA)- the main drug and medical devices regulatory body in the U.S., approved Kamada Pharmaceuticals- a global biopharmaceutical company’s application to manufacture Cytogam (cytomegalovirus immune globulin intravenous injection) at its facility located in Israel to treat COVID-19.
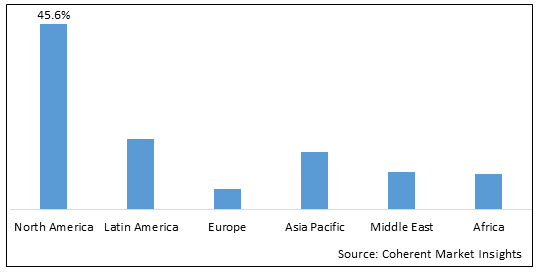
Figure 2. Global Human Immunoglobulin (pH4) for Intravenous Injection Market Share (%), by Region, 2025
To learn more about this report, Download Free Sample
Global Human Immunoglobulin (pH4) for Intravenous Injection Market - Regional Analysis
Among regions, North America is estimated to hold a dominant position in the global human immunoglobulin ph4 for intravenous injection market over the forecast period. This is due to the prevalence of COVID-19 in the region. For instance, according to a report published by WHO on December 2020, there had been 10,336,829 confirmed cases of COVID-19 with 1,127,152 deaths in 2020.
Global Human Immunoglobulin (pH4) for Intravenous Injection Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others are facing problems with the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a positive impact on the global human immunoglobulin ph4 for intravenous injection market. This is because of the rising shortages of COVID-19 drugs globally which in turn, caused researchers to develop novel immunoglobin therapies to counter the increasing demand. For instance, according to an article published in March 2022 in PubMed- a free search engine for biological databases, Italian Medicines Agency- the public institution responsible for the regulatory activity of pharmaceuticals in Italy, passed a warning on the high shortage of azithromycin in the country owing to the unprecedented prescribing of the drug during the pandemic.
Human Immunoglobulin (pH4) for Intravenous Injection Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 78.37 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 12.8% | 2032 Value Projection: | USD 182.1 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Takeda Pharmaceutical Company Limited, Baxter, CSL, Bayer AG, Grifols, S.A, Octapharma Brasil Ltda, Shanghai RAAS Blood Products Co Ltd, Hualan Biological Engineering, Inc, Top Bio Group Co, Ltd (A subsidiary of China Biologic Products Holdings, Inc), China Resources Boya Bio-pharmaceutical Group Co Ltd, ADMA Biologics, Inc, Sinopharm Group Co. Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Human Immunoglobulin Ph4 For Intravenous Injection Market Segmentation:
The global human immunoglobulin ph4 for intravenous injection market report is segmented into product type, interface, end user, and region.
By Product Type, the market is segmented into IgG, IgA, and IgM. Out of which, the igG segment is expected to hold a dominant position in the global human immunoglobulin ph4 for intravenous injection market during the forecast period and this is attributed to their higher affinity and ability to neutralize toxins, viruses, and bacteria.
By Disease Indication, the market is segmented into COVID-19, Primary Immunodeficiency Disease, Immune-mediated Thrombocytopenia, Kawasaki Disease, B Chronic lymphocytic Leukemia(B-CLL), and Others. Out of which, the COVID-19 segment is expected to hold a dominant position in the global human immunoglobulin ph4 for intravenous injection market during the forecast period and this is attributed to the rising cases of coronavirus across the globe.
By Distribution Channel, the market is segmented into Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies. Out of which, the Hospital Pharmacies segment is expected to dominate the market over the forecast period and this is attributed to the growing number of hospitalizations of patients to treat COVID-19 during the pandemic.
By Region, the market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa. Out of which North America segment is expected to dominate the market owing to the highest prevalence of COVID-19 in this region.
Among all the segmentations, the disease indication segment has the highest potential due to the increasing cases of COVID-19 across the world. For instance, according to the data published by The World Health Organization- a specialized agency of the United Nations responsible for international public health, on May 4, 2023, nearly 2.8 million new cases of coronavirus and over 17, 000 COVID-related deaths were reported between 3 to 30 April 2023.
Global Human Immunoglobulin Ph4 For Intravenous Injection Market Cross Sectional Analysis:
Among product type, IgG segment is expected to dominate in North America region during the forecast period owing to the reliance of patients on IgG Therapy for treating various illnesses. For instance, according to data published in December 2022, in PubMed- a free search engine for biological databases and medical reports, over 40% of adults with generalized myasthenia gravis (gMG) in the U.S. who had been prescribed a treatment of intravenous immunoglobulin (IVIG), became frequent users with an average of 6 doses administered each year.
Global Human Immunoglobulin Ph4 For Intravenous Injection Market : Key Developments
In July 2022, Takeda Pharmaceutical Company Limited- a Japan-based multinational pharmaceutical company, announced positive results from phase 3 clinical trial evaluating HYQVIA (Immune Globulin Infusion 10% IVIG with Recombinant Human Hyaluronidase) for maintenance treatment of Chronic Inflammatory Demyelinating Polyradiculoneuropathy (CIDP).
According to an article published in October 2022, in PubMed- a medical database for doctors and healthcare professionals, a 16-week trial conducted in dermatomyocitis patients in 2022 showed that IVIG enabled better improvement in disease condition in comparison to placebo.
In May 2020, The Food and Drug Administration (FDA) approved Octapharma Brasil Ltd a- a Switzerland-based pharmaceutical company’s Investigational New Drug Application (IND) allowing the company to initiate a phase 3 trial of Octagam (immune globulin intravenous [human]) in patients with severe coronavirus disease progression.
Global Human Immunoglobulin Ph4 For Intravenous Injection Market: Key Trends
In April 2020, Kamada Pharmaceuticals- a global biopharmaceutical company, and Kedrion S.p.A- a company that develops and distributes plasma-derived medicinal products, partnered to develop, manufacture and distribute a human plasma-derived polyclonal immunoglobulin (IgG) product for potential COVID-19 treatment. Under the collaboration, Kedrion S.p.A will collect and provide plasma at its KEDPLASMA centers from recovered Covid-19 patients. Kamada will carry out product development, manufacturing, and clinical development, in collaboration with Kedrion S.p.A.
In November 2020, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for the investigational monoclonal antibody therapy bamlanivimab by Lilly- a U.S.-based pharmaceutical company, for the treatment of mild-to-moderate COVID-19 in adult and pediatric patients. Bamlanivimab is a monoclonal antibody that is specifically directed against the spike protein of SARS-CoV-2, designed to block the virus’ attachment and entry into human cells.
Global Human Immunoglobulin Ph4 For Intravenous Injection Market: Restraints
The inefficiency of immunoglobulins to treat COVID- 19
The ineffectiveness of immunoglobin therapies can restrain the growth of the global human immunoglobulin ph4 for intravenous injection market. For instance, a study conducted on January 2022, by The National Institute of Allergy and Infectious Diseases (NIAID, part of the National Institutes of Health), concluded that adding anti-coronavirus hIVIG to a remdesivir regimen did not improve the health of a subset of adults hospitalized with COVID-19.
Test and trial in non-hospitalized patients and more investigations about the drug regimen can serve as an effective counterbalance to determine the actual efficacy of immunoglobins.
Lack of approval from WHO
The World Health Organization's recommendation against the use of ph4 Immunoglobins to treat coronavirus can restrain the growth of this market. For instance, according to the data published by WHO in December 2022, it was unknown whether IVIG products derived from pooled donor plasma contain high titers of SARS-CoV-2 neutralizing antibodies and the immunomodulatory effects of IVIG preparations, do not appear to benefit patients with COVID-19.
This can be overcome by using better substitutes and alternatives which have already been approved as an effective treatment against COVID-19.
Global Human Immunoglobulin Ph4 For Intravenous Injection Market - Key Players
Major players operating in the global human immunoglobulin ph4 for intravenous injection market include Takeda Pharmaceutical Company Limited, Baxter, CSL, Bayer AG, Grifols, S.A, Octapharma Brasil Ltda, Shanghai RAAS Blood Products Co Ltd, Hualan Biological Engineering, Inc, Top Bio Group Co, Ltd (A subsidiary of China Biologic Products Holdings, Inc), Sichuan Yuanda Shuyang Pharmaceutical Co., Ltd, Sichuan Yuanda Shuyang Pharmaceutical Co., Ltd., China Resources Boya Bio-pharmaceutical Group Co Ltd, ADMA Biologics, Inc, Sinopharm Group Co. Ltd.
*Definition: Intravenous immunoglobulin (IVIG) contains the pooled immunoglobulin G (IgG) immunoglobulins from the plasma of approximately a thousand or more blood donors. IVIGs are sterile, purified IgG products manufactured from pooled human plasma and typically contain more than 95% unmodified IgG, which has intact Fc-dependent effector functions and only trace amounts of immunoglobulin A (IgA) or immunoglobulin M (IgM).
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients